Abstract
Background/Objectives
The prevalence of myopia is higher in preterm infants who underwent laser photocoagulation (LPC) for retinopathy of prematurity (ROP). The aim of this study was to investigate factors associated with myopia in preterm infants who undergo LPC for ROP.
Subjects/Methods
We retrospectively analysed the medical records of preterm infants born at Kyushu University Hospital (October 2008–March 2018) at ≤32 weeks of gestational age or with birth weight ≤1500 g. We evaluated the associations between nine clinical factors and the spherical equivalent at 1-year corrected age by performing multivariable linear regression in LPC-treated ROP patients.
Results
Among the 485 infants enroled, 76 developed ROP requiring treatment. Of these, 71 underwent LPC, which was provided to 63 infants as the primary treatment (LPC alone or the combination therapy of LPC and intravitreal injection of bevacizumab [IVB]) and to eight infants as additional LPC after IVB monotherapy. The results of a refractive examination at 1-year corrected age were available for 110 eyes of 56 infants (78.9%). The mean ± standard deviation of the SE value was −0.5 ± 3.0 dioptres (D). Multivariable linear regression analysis revealed a significant association between laser spot count and SE value (ß = −0.081 ± 0.040 D per 100 spots [mean ± standard error], p = 0.045).
Conclusions
Our results suggest that an increased laser spot count observed during ROP treatment associates with myopia.
Similar content being viewed by others
Introduction
Despite advances in treatment, retinopathy of prematurity (ROP) remains a leading cause of childhood blindness [1]. The current standard treatment for ROP is laser photocoagulation (LPC), whose efficacy was established by the Early Treatment for ROP (ETROP) trial [2]. However, the ETROP trial and other studies demonstrated that the prevalence of myopia increased in LPC-treated ROP patients, due to ocular structural sequelae [3,4,5,6].
On the other hand, the Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity trial showed that compared with LPC, an intravitreal injection of bevacizumab (IVB) decreased the recurrence rate of zone I ROP [7], and over the past decade the intravitreal injection of anti-vascular endothelial growth factor agents (anti-VEGF therapy) has become more common as the primary treatment for severe ROP [8]. Although anti-VEGF therapy can also improve the structural outcome and reduce the incidence of myopia compared with LPC [9, 10], approximately 10–40% of anti-VEGF-treated patients with ROP require additional treatment due to recurrence [8, 11]. The potential development of myopia in patients who have undergone additional LPC after IVB treatment must therefore be monitored.
Infants with ROP tend to develop myopia due to the arrested development of the anterior segment [12, 13]. LPC worsens these ocular structural changes, and the prevalence of myopia is significantly higher in LPC-treated ROP patients than in patients with regressed ROP [14, 15]. However, the risk factors of myopia after LPC are still controversial [16]. We conducted the present study to investigate the relationship between nine candidate factors (gestational age [GA], birth weight [BW], corrected weeks of age and body weight at the first treatment, zone, stage, laser spot count, laser wavelength, and type of primary treatment [LPC, LPC + IVB, or IVB]) and the spherical equivalent (SE) value in LPC-treated ROP patients at 1-year corrected age.
Subjects and methods
Subjects
This study was performed in accordance with the tenets of the Declaration of Helsinki. Having obtained approval from the Institutional Review Board at Kyushu University Hospital, we conducted a retrospective chart review of the infants who underwent ophthalmic examinations at the Kyushu University Hospital from October 2008 through March 2018. We presented information of this study on our institutional website and informed all parents of their right to opt out. An ROP screening examination was provided to all infants born at ≤32 weeks of GA or with a BW ≤ 1500 g. We selected the infants who had undergone LPC and whose SE results at 1-year corrected age were available for our analyses of the associations of clinical factors with SE.
Primary treatment
The ROP staging of each subject was decided based on the International Classification of Retinopathy of Prematurity Revisited [17]. LPC, LPC + IVB (0.625 mg/0.025 ml), or IVB had been performed as the primary treatment for the infants with ‘type 1 ROP’ as described in the ETROP trial (stage 2 or 3 in zone II with plus disease, stage 3 in zone I with or without plus disease, or stage 1 or 2 disease in zone I with plus disease) [2] or worse ROP. LPC was applied to the entire avascular area with half laser burn width. A diode laser (808 nm wavelength; DC-3000, Nidek, Aichi, Japan) was used for 58 eyes of 29 infants until December 2012, and a Nd-YAG laser (532 nm wavelength; MC-500, Nidek) was used for 52 eyes of 27 infants thereafter. The settings of laser ablation with 808 nm and 532 nm wavelength were set at a power of 200–400 mW for 300 msec and a power of 80–100 mW for 300 msec, respectively. Bevacizumab was injected with a 30-gauge needle 1.5 mm posterior to the limbus. LPC and IVB were provided to ROP patients requiring treatment under intravenous sedation.
Additional laser photocoagulation
Follow-up examinations after treatment were performed on a weekly or biweekly basis until the patient’s ROP regressed or vascularization reached zone III. Recurrence was defined as a new appearance of plus disease, neovascularization, ridge, or proliferative membrane. All recurrences were treated with LPC. In addition to these criteria, additional LPC was performed for IVB-treated ROP patients to prevent late recurrence [18,19,20] when an avascular area remained before the patient’s discharge or when the arrest of vascularization was accompanied by abnormal hyper-permeability observed through fluorescein angiography.
Refraction
An auto-refractometer (HandyRef, Nidek) was used to measure the refraction at 1-year corrected age. If the use of this device was not possible, the refraction was measured with manual retinoscopy. All refraction readings were obtained after the instillation of 1% tropicamide, 2.5% phenylephrine hydrochloride, and 1% cyclopentolate hydrochloride.
Statistical analysis
Using multivariable linear regression, we evaluated the association of the nine above-described factors with the SE value. In addition, in view of the effect of ROP severity on the SE value, a stratified analysis by zone was performed using Student’s t test.
All analyses were carried out using SAS software (ver. 9.3, SAS, Cary, NC). GA, BW, corrected weeks and body weight at first treatment, and laser spot count were treated as continuous variables, and the other parameters as categorical variables. Using the factors that were significant in univariable analysis, we conducted multivariable-adjusted linear regression analysis. A two-sided p value < 0.05 was considered significant.
Results
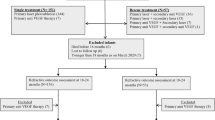
ROP screening examination was provided to 485 infants. Of these, 232 (48%) developed ROP, and 76 (16%) developed ROP requiring treatment. LPC (n = 53), LPC + IVB (n = 10), or IVB (n = 13) were provided to these infants as the primary treatment (Fig. 1). Among the 53 LPC-treated ROP patients, recurrence occurred in four (8%), while among the 10 treated with LPC + IVB, recurrence appeared in one (10%). Among the 13 ROP patients treated with IVB monotherapy, eight infants received additional LPC (recurrence, n = 5 [38%]; remained avascular area, n = 2; abnormal fluorescein leakage, n = 1). A total of 71 infants thus received LPC. Of these 71 infants, refractive examination results at 1-year corrected age in 110 eyes of 56 infants (the bilateral ROP occurred in 54 infants and the asymmetric ROP occurred in 2 infants) were available. The reasons for the exclusion of 15 patients were as follows: (1) eight patients could not be examined because of relocation, (2) five patients did not visit our department at the scheduled date, and (3) two patients died before 1-year corrected age.
Table 1 summarizes the characteristics of the 56 infants, including their GA, BW, corrected weeks and body weight at first treatment, zone, stage, laser spot count, laser wavelength, and type of primary treatment. The mean ± standard deviation of the SE value at 1-year corrected age was −0.5 ± 3.0 dioptres (D). Table 2 provides the results of the linear regression analyses. In the univariable analysis, laser spot count (ß = −0.102 ± 0.039 D per 100 shots, p = 0.009, Fig. 2) and use of 808 nm laser (ß = −1.460 ± 0.564 D, p = 0.011) were significantly associated with the SE value.
We then performed a multivariable analysis using both laser spot count and 808 nm laser use as variables. Only laser spot count was significantly related to the SE value at the patients’ 1-year corrected age (ß = −0.081 ± 0.040 D per 100 shots, p = 0.045, Table 2).
The severity of ROP is defined by a combination of zone and stage
Considering the effect of ROP severity on the SE value, we classified all eyes into zone I or zone II ROP and investigated whether the ROP stage influence the SE value. In both the zone I ROP and the zone II ROP, there was no significant difference in the SE value between stage 2 and stage 3 (p = 0.608 and p = 0.771, respectively, Table 3).
Discussion
Our findings provide the first demonstration that laser spot count during ROP treatment is significantly associated with myopia, based on a quantification of the influence of laser spot count on the SE value. Our observation of a significant correlation between laser spot count and SE value suggests that laser scarring caused ocular structural sequelae (e.g., a thinner anterior chamber depth and a steeper corneal curve) which could promote myopia [16]. This finding is consistent with the results of a study showing that laser spot counts were significantly larger in infants with myopia (SE value ≤ −0.25 D) than in those without myopia (SE value > −0.25 D) [21].
It is known that the more severe the ROP, the greater the myopia, even if untreated [22]. In Table 2, neither zone nor stage were shown to affect the SE value alone, but the severity of ROP is defined by a combination of zone and stage. The stage of the infants analysed in this study was either (1) zone I, stage 2 with plus, (2) zone I, stage 3 with plus, (3) zone II, stage 2 with plus, (4) zone II, stage 3 with plus. According to the ROP activity scale [23], all of these are classified as “severe,” so there is no difference in severity. To confirm the impact of ROP severity on the SE value, a stratified analysis by zone was performed. However, there was no significant difference in the SE value between stage 2 and stage 3. These results suggest that laser spot count is an independent risk factor for myopia.
In addition, a marginally significant correlation between the use of the 808 nm laser and myopia was found in the present study. However, we cannot conclude that a 532 nm laser is better than an 808 nm laser. The effect size of both laser spot count and 808 nm-laser use decreased in the multivariable-adjusted analysis, implying that these factors are not completely independent. Indeed, there was a significant difference in laser spot count between patients treated with 532 nm versus 808 nm lasers in the present study (532 nm = 1264 ± 520 shots, 808 nm = 1677 ± 838 shots [mean ± standard deviation], p = 0.002, t test). LPC was performed at half-width in all patients, but there was a significant difference in the number of ablations. It was reported that 532 nm laser spots on the retina are more easily observed than 808 nm laser spots [15]. We therefore speculate that the infants who underwent LPC with the 808 nm laser may have received a greater number of photocoagulations than necessary.
An 808 nm laser is considered more effective than 532 nm laser in ROP treatment because of its ability to burn the deeper layer of the retina [15, 16]. An 808 nm laser also has the advantage of reducing the risk of developing cataract [24]. In our population, cataract occurred only in the 532 nm laser group (1 in 52 cases [2%]), although not so severe as to require lensectomy. However, an 808 nm laser has been reported to be more at risk of promoting myopic shift than a shorter wavelength laser [25]. In addition, several reports revealed that a 532 nm or 659 nm laser has therapeutic effects equal to those of an 808 nm laser [26, 27]. Since myopia and cataract are major complications related to vision prognosis, we suggest that further investigations are needed to study the effects of laser wavelength on therapeutic efficacy and incidence of complications.
The limitations of this study are as follows. (1) Its retrospective nature made it difficult to determine the causal relationship between laser spot count and myopia. (2) The relative proportion of severe ROP cases requiring treatment has increased due to the improvement in the survival rate of preterm infants [28, 29]. Differences in the general status of premature infants might cause bias deriving from factors related to neonatal management. (3) The laser wavelength was changed during the study enrolment period, and we did not treat ROP patients with different wavelengths in the same period. (4) LPC was performed by a total of five ophthalmologists. Indeed, only one ophthalmologist was involved in the ROP treatment for the entire period, and each of the other four used either the 532 nm laser only or the 808 nm laser only. Considering that the laser spot count required for treatment can be expected to differ depending on the skill level of the ophthalmologist, this might have caused differences in laser spot counts.
In conclusion, our analyses revealed a significant association between laser spot count and SE value in LPC-treated patients with ROP. Preterm infants who have received many laser shots may be at risk of developing myopia at 1-year of corrected age.
Summary
What was known before
-
LPC is the standard treatment for ROP
-
The prevalence of myopia is higher in ROP patients who received LPC
What this study adds
-
An increased number of laser spots is related to myopia following LPC for ROP
-
Further investigation is needed on the effects of laser wavelength on therapeutic efficacy and incidence of complications
References
Shah PK, Prabhu V, Karandikar SS, Ranjan R, Narendran V, Kalpana N. Retinopathy of prematurity: Past, present and future. World J Clin Pediatr. 2016;5:35–46.
Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group. final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–50.
Ling CS, Fleck BW, Wright E, Anderson C, Laing I. Diode laser treatment for retinopathy of prematurity: Structural and functional outcome. Br J Ophthalmol. 1995;79:637–41.
Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM, et al. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology. 2005;112:1564–8.
Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, et al. progression of myopia and high myopia in the early treatment for retinopathy of prematurity study. findings to 3 years of age. Ophthalmology. 2008;115:1058–.e1.
Dhawan A, Dogra M, Vinekar A, Gupta A, Dutta S. Structural sequelae and refractive outcome after successful laser treatment for threshold retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2009;45:356–61.
Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15.
VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:619–33.
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity. Ophthalmology. 2015;122:1008–15.
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: A randomized clinical trial. JAMA Ophthalmol. 2014;132:1327–33.
Huang Q, Zhang Q, Fei P, Xu Y, Lyu J, Ji X, et al. Ranibizumab injection as primary treatment in patients with retinopathy of prematurity: Anatomic outcomes and influencing factors. Ophthalmology. 2017;124:1156–64.
Fielder AR, Quinn GE. Myopia of prematurity: Nature, nurture, or disease? Br J Ophthalmol. 1997;81:2–3.
O’Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ, Ratib S, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–18.
Yang CS, Wang AG, Shih YF, Hsu WM. Long-term biometric optic components of diode laser-treated threshold retinopathy of prematurity at 9 years of age. Acta Ophthalmol. 2013;91:e276–e282.
Chan-Ling T, Gole GA, Quinn GE, Adamson SJ, Darlow BA. Pathophysiology, screening and treatment of ROP: A multi-disciplinary perspective. Prog Retin Eye Res. 2018;62:77–119.
Houston SK, Wykoff CC, Berrocal AM, Hess DJ, Murray TG. Laser treatment for retinopathy of prematurity. Lasers Med Sci. 2013;28:683–92.
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Ittiara S, Blair MP, Shapiro MJ, Lichtenstein SJ. Exudative retinopathy and detachment: A late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J Aapos. 2013;17:323–5.
Mataftsi A, Koulali E, Papageorgiou E, Ziakas N, Brazitikos P. Letter to the Editor: Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging Retin. 2017;48:372.
Hajrasouliha AR, Garcia-Gonzales JM, Shapiro MJ, Yoon H, Blair MP. Reactivation of retinopathy of prematurity three years after treatment with bevacizumab. Ophthalmic Surg Lasers Imaging Retin. 2017;48:255–9.
Katoch D, Sanghi G, Dogra MR, Beke N, Gupta A. Structural sequelae and refractive outcome 1 year after laser treatment for type 1 prethreshold retinopathy of prematurity in Asian Indian eyes. Indian J Ophthalmol. 2011;59:423–6.
Larsson EK, Rydberg AC, Holmström GE. A population-based study of the refractive outcome in 10-year-old preterm and full-term children. Arch Ophthalmol. 2003;121:1430–6.
Smith LE, Hellström A, Stahl A, Fielder A, Chambers W, Moseley J, et al. Development of a retinopathy of prematurity activity scale and clinical outcome measures for use in clinical trials. JAMA Ophthalmol. 2019;137:305–11.
McNamara JA. Laser treatment for retinopathy of prematurity. Curr Opin Ophthalmol. 1993;4:76–80.
Roohipoor R, Karkhaneh R, Riazi Esfahani M, Alipour F, Haghighat M, Ebrahimiadib N, et al. Comparison of refractive error changes in retinopathy of prematurity patients treated with diode and red Lasers. Ophthalmologica. 2016;235:173–8.
Sanghi G, Dogra MR, Vinekar A, Gupta A. Frequency-doubled Nd:YAG (532 nm green) versus diode laser (810 nm) in treatment of retinopathy of prematurity. Br J Ophthalmol. 2010;94:1264–5.
Chhabra K, Kaur P, Singh K, Aggarwal A, Chalia D. Outcome of solid-state 532 nm green laser in high-risk retinopathy of prematurity at a tertiary care centre in India. Int Ophthalmol. 2018;38:287–91.
Dhingra D, Katoch D, Dutta S, Samanta R, Aggarwal K, Dogra MR. Change in the incidence and severity of retinopathy of prematurity (ROP) in a neonatal intensive care unit in Northern India after 20 years: Comparison of two similar prospective cohort studies. Ophthalmic Epidemiol. 2019;26:169–74.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–51.
Acknowledgements
This study was supported by a grant from Japan’s MEXT KAKENHI (No. 19K18846) to MA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mori, Y., Arima, M., Ueda, E. et al. Risk factors for myopia at 1-year corrected age following laser photocoagulation for retinopathy of prematurity. Eye 35, 2820–2825 (2021). https://doi.org/10.1038/s41433-020-01321-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01321-z
This article is cited by
-
Development of myopia in laser-treated ROP infants: prematurity or laser photocoagulation?
International Ophthalmology (2022)
-
Risk factors for early-onset high myopia after treatment for retinopathy of prematurity
Japanese Journal of Ophthalmology (2022)