Abstract
Beta-blockers have been associated with decreased cancer mortality. However, evidence for lung cancer is sparse and reported beneficial effects might be based on biased analyses. In this so far largest study we investigated the association between β-blocker use and lung cancer survival. Therefore, patients with a lung cancer diagnosis between April 1998 and December 2011 were selected from a database linkage of the Netherlands Cancer Registry and the PHARMO Database Network. After matching eligible patients on the propensity score, adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CI) were calculated using Cox proportional hazards regression to investigate the association between pre-diagnostic and time-dependent β-blocker use and overall survival. Duration and dose-response analyses and stratified analyses by β-blocker type, histological subgroups and stage were conducted. Of 3,340 eligible lung cancer patients, 1437 (43%) took β-blockers four months prior to diagnosis. Pre-diagnostic β-blocker use was not associated with overall survival (HR 1.00 (0.92–1.08)) in the adjusted model. Time-dependent post-diagnostic analysis showed similar results with a HR of 1.03 (0.94–1.11). Trend analyses showed no association for cumulative dose (HR 0.99 (0.97–1.02)) and cumulative duration (HR 1.00 (0.96–1.05)). In conclusion, β-blocker use is not associated with reduced mortality among lung cancer patients.
Similar content being viewed by others
Introduction
With about 1.8 million new cases in 2012 lung cancer is the cancer type with the highest incidence worldwide1. Moreover, the prognosis for this disease still remains very poor and lung cancer represents the most common cause of death from cancer overall. Many previous experimental and epidemiological findings suggested that an upregulated activity of the sympathetic nervous system and cancer-related stress responses might lead to enhanced metastatic involvement and tumor growth which could be antagonized by β-adrenergic receptor blockade2,3,4.
Therefore, particularly β-blockers were proposed as a new add-on treatment for several cancer types. This hypothesis attracted much attention recently when propranolol, a nonselective β-blocker, was introduced as the new first-line treatment for infantile hemangiomas5, 6. However, so far only four observational studies7,8,9,10, with not more than a few hundred patients each, and two screening studies11, 12 investigated the association between β-blocker use and prognosis after lung cancer. Besides inconsistent results for non-small cell lung cancer (NSCLC), which were ranging from a protective to no association, no results have been published so far on small cell lung cancer (SCLC).
Additionally, the results of these studies are misleading as the analyses were found to be incorrect involving immortal time bias and insufficient confounder adjustment for potentially important prognostic factors13, 14.
Given that β-blockers are widely used for several indications and are considered as safe, effective, and well-established in routine care, benefits for lung cancer patients would be of utmost interest. Hence, these analyses aim to investigate the hypothesis whether concomitant β-blocker use is associated with a survival benefit among both NSCLC and SCLC patients. Including a population of 7,002 lung cancer patients, which is exceeding the study size of all previous studies taken together (N = 6,178), we provide results from the so far largest population-based study on the association between β-blocker use and lung cancer prognosis.
Methods
Data sources
The data used for this retrospective population-based cohort study comprises a comprehensive database linkage of the Netherlands Cancer Registry (NCR) and the PHARMO Database Network15. Data from the Eindhoven area of the NCR were used which covers a demographic region with 2.4 million inhabitants. The Eindhoven cohort of the NCR is a population-based cancer registry which collects information on patient and tumor characteristics, co-morbidities, and socio-economic status. Vital status is obtained by linkage to Dutch municipal records. The PHARMO Database contains longitudinal data gathered from community pharmacies including information on drug dispensing records, units and packages supplied, dose descriptions, and detailed information on active ingredients according to their Anatomical Therapeutic Chemical/Defined Daily Dose Classification (ATC/DDD) code16.
Study population
Patients were eligible if they had a diagnosis of lung cancer between 1st April 1998 and 31st December 2011. Only primary lung cancers (with and without pathological confirmation) were included. Patients with missing information on important prognostic or risk factors were excluded. Due to the high model complexity of our statistical analyses and the sufficient amount of study participants, we refrained from using multiple imputation approaches to account for missing data. To mitigate healthy-user effects and confounding by indication we restricted the analysis cohorts to patients taking β-blockers or guideline medications prescribed alternatively to β-blockers during the four-month period prior to diagnosis (active comparators) as this was shown to lead to more unbiased results (Supplementary Table 1)17. Out of this population, a propensity score matched cohort was created to conduct all analyses in a quasi-experimental cohort setting, simulating equally distributed baseline factors.
Classification and modelling of medication use
Patients were classified as β-blocker users if they received at least one β-blocker dispensing from the ATC code group C07 in a four-month interval. This interval was chosen, because an explorative analysis of the dataset has shown a more accurate representation of time-dependent drug utilization compared to a usually used three-month interval.
Beta-blocker subgroups according to their β1-receptor affinity (selective, nonselective) and single active ingredients were determined for subgroup analyses. As β-blockers were also shown to have variable tissue availability, subgroup analyses were additionally performed stratified by their pharmacokinetic properties (hydrophilicity/lipophilicity)18. To adjust for potential confounding effects by other medication classes, dispensed non-steroidal anti-inflammatory drugs (NSAIDs), statins, diabetes medications, antihypertensive treatments, and other medications with indications similar to those for β-blockers were also considered (Supplementary Table 1).
Use of β-blockers was once investigated as pre-diagnostic use and once as a time-varying covariate in post-diagnostic analyses. For pre-diagnostic analyses, β-blocker use was modelled as a time-fixed covariate, classifying patients as pre-diagnostic users if they received at least one β-blocker dispensing four months prior to diagnosis (yes/no). To overcome immortal time bias, the use of β-blockers after lung cancer diagnosis (post-diagnostic use) was modelled as a time-varying covariate according to the Mantel-Byar method, that is, patients were initially considered non-users and then users after a lag of four months after their first post-diagnostic β-blocker dispensing until death or end of follow-up19,20,21. To avoid time-varying confounding and selection bias a first-treatment-carried-forward approach was used to model treatment changes (intention-to-treat analysis)22, 23. The additional lag of four months was introduced to mitigate possible sick-stopper effects and to account for a biologically reasonable latency window and reverse causality22, 24. Given the information from the cancer registries that missing information on comorbidity will probably be more often seen among those without comorbidity, we additionally conducted sensitivity analyses coding missing information as having no comorbidity.
In post-diagnostic analyses, cumulative duration and cumulative dose were also investigated time-dependently starting with at least one dispensing four months prior to diagnosis. Cumulative duration of use was defined according to the following categories: 1–12 months, 13–24 months, 25–36 months and >36 months. The DDD of each dispensing was calculated by multiplying the dispensed number of tablets by the dose, divided by the DDD classification of the World Health Organization16 and were then categorized as 0 DDDs, >0–365 DDDs and 366+ DDDs. If doses were missing the dose from the previous treatment interval was used.
Statistical analysis
The distribution of the basic characteristics of all eligible patients and the propensity score matched patients were compared between β-blocker users and active comparators based on β-blocker use four months prior diagnosis applying Pearson’s χ2 test.
The propensity score matched cohort was created by using logistic regression to calculate propensity scores, that is, the probability (propensity) of an individual receiving the treatment based on the patient’s observed time-invariant pre-treatment baseline variables as proposed by Rosenbaum and Rubin25. The following potential baseline prognostic and risk factors were included in the model: age, sex, year of diagnosis, socio-economic status (low, middle, high, institutionalized), comorbidities (cardiovascular, hypertension, cerebrovascular, lung, diabetes), treatment (surgery, chemotherapy, radiotherapy, radiotherapy aimed at metastasis), best supportive care, stage (UICC), histology (NSCLC, SCLC, other), previous cancer, baseline medication use and number of distinct ATC classes prescribed during four months prior to diagnosis26. Individuals were subsequently matched using a 1:1 nearest-neighbor matching algorithm with a caliper width of 0.2 standard deviations of the propensity score logit and without replacement as suggested by simulation studies27,28,29. Nearest neighbor matching was performed using the %match_NearestNeighborMatch macro provided by Rassen and Schneeweiss et al.30. To account for the matched nature of the sample (clustering in matched pairs), a robust (sandwich co-) variance (matrix) estimator that accounts for the clustering within matched sets was used31, 32.
Adjusted HRs, estimated by Cox proportional hazard regression, were used to assess the association between pre- and post-diagnostic β-blocker use and overall survival. Time-varying use of concomitant medication after diagnosis was included in the model to adjust for time-dependent effects. To account for the matched nature of the sample, a robust variance estimator that accounts for the clustering within matched sets was used31, 32. Follow up time was calculated using the reverse Kaplan-Meier method from date of diagnosis until death, migration from the NCR-PHARMO catchment area or end of study period (31st December 2013), whichever occurred first.
Subgroup analyses were conducted by β-blocker subtype, histological subtypes and stage. As none of the previous studies considered active comparators as their reference group and propensity score matching in their analysis, we repeated all analyses with non-users as comparison group and conventional Cox regression without propensity score matching. Results from these analyses (presented in Supplementary Tables) have to be interpreted with caution, however, as they might be more prone to bias17.
The proportional hazards assumption was assessed by including a time-dependent component for each explanatory variable in the Cox model. For pre- and post-diagnostic use, the proportional hazards assumption was violated by treatment and stage covariates. As HRs did not meaningfully change when allowing these parameters for time-varying effects, we refrained from including these factors in the main analyses to keep the model complexity low.
All analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) according to an a-priori defined study protocol. Statistical significance was defined by a two-sided P < 0.05.
Data availability
The dataset analyzed during the current study is a database linkage which is not publicly available and is licensed to be analyzed for the investigated research question only.
Results
Study population and medication use at baseline
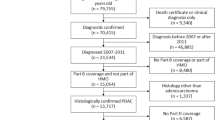
Out of 7002 lung cancer patients, 3340 patients (47.7%) were eligible to be included in the study either as β-blocker user or active comparator (Fig. 1).
The majority of patients were male (73%), diagnosed with NSCLC (71%) and had TNM stage III or IV (66%). Median follow-up time was 6.5 years (25th percentile: 4.1 years, 75th percentile: 9.5 years). During follow-up 2967 (89%) patients died, 2079 (70%) of them within the first 12 months.
Within the four months prior to diagnosis, 1437 (43%) of all patients took β-blockers. Selective β-blockers were used more frequently (38%) than nonselective β-blockers (5%) among all eligible patients. Metoprolol was the active ingredient used most (20%), followed by bisoprolol and atenolol (each 8%) and sotalol (3%). Restricting the analysis cohort to active comparators (before propensity score matching) already resulted in a more balanced cohort than simply comparing to non-users (Table 1). Βeta-blocker user and active comparators only differed significantly in the year of diagnosis, in the presence of comorbidities, use of other antihypertensives, use of statins and number of distinct medication classes dispensed.
After matching users and active comparators on propensity scores, 2500 patients remained for statistical analysis (Fig. 1). The assessment of distributions of baseline variables showed that the propensity score matching led to a well-balanced cohort for all measured baseline characteristics (Table 1).
Pre-diagnostic β-blocker use and survival
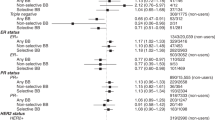
After adjustment for relevant covariates in multivariable analyses, no associations were observed for any β-blocker use (1.00 (0.92–1.08)) (Table 2 and Fig. 2).
For β-blocker subgroups there were significant associations for nonselective β-blocker use (1.22 (1.01–1.46)). Analyses stratified by stage and site showed significant associations for stage III patients using selective β-blockers (0.85 (0.73–0.99)). However, HRs for stage IV for any β-blocker use, selective β-blocker use and SCLC in the nonselective β-blocker group were increased (1.20 (1.06–1.35), 1.18 (1.04–1.34) and 1.67 (1.14–2.46), respectively).
When repeating the analysis with non-users as reference group, HRs were partially higher but followed the same trend (0.99 (0.92–1.07) and 1.00 (0.93–1.09) for any β-blocker use) (Supplementary Table 2).
Post-diagnostic β-blocker use and survival
The results for β-blocker use modelled as a time-varying covariate in time-dependent analyses are presented in Table 3.
Overall there was again no evidence for an association for any β-blockers (1.03 (0.94–1.11)). There was also no significant effect amongst all β-blocker subgroups besides an increase in mortality for nonselective β-blockers (1.26 (1.06–1.49)).
In stage and site specific analyses almost all results were clustered around the null. There was just a slight decrease in mortality for stage IV patients taking any (1.17 (1.03–1.33)) or selective (1.15 (1.01–1.30)) β-blocker and SCLC patients taking nonselective β-blocker (1.79 (1.27–2.51)).
When repeating the analysis without active comparison, estimates were again higher leading to a slight increase in hazard ratios for all eligible patients (1.09 (1.02–1.17)) while in a propensity score setting with non-users as reference there was still no significant association (1.03 (0.95–1.12)) (Supplementary Table 3).
Cumulative duration and cumulative dose
Results from cumulative dose-response and duration analyses are shown in Table 4.
In general, also dose and duration specific analyses did not show much evidence for an association. An increase in mortality for doses between 0–365 DDDs of nonselective β-blockers was observed (1.33 (1.11–1.59)), whereas the HR for a cumulative duration of 13–24 months of selective β-blocker use (0.83 (0.70–0.98)) was decreased.
Comparing β-blocker use to non-users led again to much higher estimates as presented in Supplementary Table 4.
Discussion
In this so far largest population-based study addressing the association of β-blocker use and lung cancer survival, we found no clinically relevant evidence for a survival benefit of pre- or post-diagnostic β-blocker use among lung cancer patients. There were some significant associations when stratifying for β-blocker subtypes, stage, site, dose or duration of use but they did not follow a consistent direction.
Results from previous studies on the association of β-blocker use and lung cancer survival were inconclusive and some of them are suspected to have reported too overoptimistic results which might be due to factors like immortal time bias14. So far, two studies have suggested a beneficial use of β-blockers for NSCLC patients. The latest study from 2015 suggested a 22% decrease in mortality, but was only based on hospital-based data from 673 patients with stage III NSCLC9. Another study by the same authors from 2013 showed similar results for distant metastasis-free survival, disease-free survival, and overall survival10.
In contrast, two other studies on lung cancer survival rather proposed no association between β-blockers and overall survival7, 8. However, also these two studies with a total of 107 and 435 patients, respectively, were both hospital-based and therefore limited to data ascertained from monocentric medical chart reviews. Additionally, in a recently published meta-analysis focusing on β-blocker use and cancer prognosis with special emphasis on immortal time bias, these studies were both suspected to have incorporated immortal person-time which further limits the interpretability of their findings14. Furthermore, two population-based screening studies including lung cancer patients reported no association between β-blocker use and overall survival after lung cancer with hazard ratios comparable to ours (1.01 (0.93–1.11) and 1.12 (0.89–1.41), respectively)11, 12.
All of our main results in both pre- and post-diagnostic analyses very precisely and consistently suggest that β-blocker use might not be associated to overall survival among lung cancer patients. However, subgroup analyses showed some inconsistent significant results which might be due to the following reasons.
Firstly, it is very likely that the majority of these results might be due to chance and distorted by other factors like small subgroup sample sizes and residual confounding caused by unmeasured covariates. Without adjustment for confounding, we generally observed higher HRs which decreased after comprehensive covariate adjustment in a sensitivity analysis (data not shown). Secondly, we performed a large number of statistical tests in stratified analyses which always creates the potential of statistical significance due to chance. The fact that we observed these significant results in both directions strengthens this assumption. Thirdly, many of these inconsistent findings were calculated in subgroups with low sample sizes like nonselective β-blockers, which are only rarely prescribed in clinical practice for a longer period of time.
Strengths and limitations
This study has potential limitations. Although our calculations are based on a comprehensive database, we still excluded some patients with missing information, mainly on variables containing data on co-morbidity. We also refrained from imputing our missing data to conduct a complete case analysis. Coding patients with missing information on comorbidity as having no comorbidity in sensitivity analysis, however, did not alter the results (data not shown).
Additionally, antiangiogenic mechanisms were proposed to be mediated via β2-adrenoceptor blockade suppressing cAMP levels and activating extracellular signal-regulated kinase (EKR)1/2 in a dose dependent way6. However, our sample sizes for analyses involving only nonselective β-blockers, which are able to block β2-adrenoceptors, was small compared to the ones with β1 selective β-blockers.
Another limitation is the use of overall survival as our main outcome. As we had no information on the cause of death, we were not able to calculate lung cancer-specific survival or to investigate competing risks. However, given the poor prognosis of a lung cancer diagnosis, it is very likely that the majority of patients died due to their lung cancer disease. Nevertheless, overall survival needs to be interpreted carefully on the basis of the underlying medication use and co-morbidities. We addressed this problem by restricting the reference group to active comparators which led to equal cohorts in terms of health-seeking behaviors and frailty and therapy adherence. Additionally we accounted for this by adjusting for comorbidities, time-varying treatment and distinct numbers of medications used, as this was shown to have a good performance as a comorbidity measure to control for confounding26. Unfortunately, dose-responses could only be approximated by DDDs as no operationalisable variable was available to measure real drug utilization. This information could have also been of interest for assessment of therapy compliance.
However, our study also has unique strengths. To our knowledge this is the largest and most comprehensive population-based cohort study which has been conducted so far focusing on the association of β-blocker use and lung cancer mortality. A power analysis indicated that a harmful or protective association with HRs ≥ 1.13 or HRs ≤ 0.89, respectively, could have been detected in our main analysis with a significance level of α = 5% and a power of 80% (β = 0.2)33. For subgroup analyses only stronger associations could have been detected, however, (e.g. HR ≥ 1.45/≤0.69, ≥1.70/≤0.59, ≥1.24/≤0.81, ≥1.20/≤0.83 in stage specific analyses for stage I, II, III and IV cancers, respectively). Nevertheless, we were able to address key exposure characteristics, such as tissue availability of β-blockers (influenced by physico-chemical properties such as lipophilicity), pharmacodynamic characteristics (receptor selectivity), cumulative treatment duration, and dose as well as key tumor characteristics such as stage and histological subtypes. Also tumor stage was addressed as an important factor because angiogenic markers appear to have more prognostic value in earlier stages of the disease34 and probably rather in NSCLC than in SCLC35.
In contrast to the majority of the previously published studies, our analysis was based on data from a population-based cohort, which allows for a more generalizable interpretation of the association of β-blocker use and lung cancer prognosis than in monocentric hospital-acquired data. In addition to the high quality and validity of the underlying data we were able to conduct a comprehensive confounder adjustment, which was shown to be of outstanding importance in pharmacoepidemiological studies15, 36.
Because several previous studies did not consider time-dependent treatment effects, making them prone to a variety of biases, especially immortal time bias14, we carefully addressed this issue by modelling post-diagnostic β-blocker use according to the Mantel-Byar method as simulations studies demonstrated that this is the gold-standard for time-dependent modelling13, 21.
As shown in previous studies, it is also of importance to address paradoxical relations of drug treatment in elderly populations in terms of sick-stopper effects, which means that moribund patients are likely to discontinue preventive medication for, in this case, non-lung cancer related conditions22. Hence, a strength of our analysis is that we accounted for these end-of-life treatment effects with a first-treatment-carried-forward approach (intention-to-treat analysis) and an additional four-month lag after a patient’s first β-blocker dispensing to avoid spurious findings due to reverse causality and informative censoring.
Conclusion and implications for clinical practice
Βeta-blockers are well-established and indisputably valuable drugs for managing cardiovascular diseases, hypertension, heart failure, primary migraine prophylaxis, and essential tremor. The effectiveness of propranolol in the treatment of infantile hemangiomas, which is assumed to be based on antiangiogenic actions mediated by the β2-adrenoceptor6, might encourage further studies to concentrate on larger patient populations receiving non-selective β-blocker therapy, tumor entities and stages34 for which angiogenesis has a high impact on tumor progression37,38,39. Utilization of β-blockers as an add-on therapy in cancer treatment would be of utmost interest not only to inhibit metastatic spread and cell growth, but also for public health implications. However, our study does not support such use for patients with lung cancer.
In conclusion, after careful interpretation of our data and taking further sensitivity analyses and possible residual confounding into account, we did not find evidence for a beneficial role of pre- or post-diagnostic β-blocker use for lung cancer patients in this so far largest and most comprehensive study. More evidence and a final conclusion will have to come from currently ongoing randomized clinical trials40,41,42,43,44.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–386, doi:10.1002/ijc.29210 (2015).
Cole, S. W. & Sood, A. K. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin. Cancer. Res. 18, 1201–1206, doi:10.1158/1078-0432.ccr-11-0641 (2012).
Entschladen, F., Drell, T. Lt., Lang, K., Joseph, J. & Zaenker, K. S. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 5, 254–258, doi:10.1016/s1470-2045(04)01431-7 (2004).
Partecke, L. I. et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology, doi:10.1016/j.pan.2016.03.005 (2016).
Leaute-Labreze, C. et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N. Engl. J. Med. 372, 735–746, doi:10.1056/NEJMoa1404710 (2015).
Munabi, N. C. et al. Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation. Stem Cells Transl. Med 5, 45–55, doi:10.5966/sctm.2015-0076 (2016).
Aydiner, A., Ciftci, R., Karabulut, S. & Kilic, L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac. J. Cancer Prev. 14, 6109–6114 (2013).
Cata, J. P. et al. Perioperative beta-blocker use and survival in lung cancer patients. J. Clin. Anesth. 26, 106–117, doi:10.1016/j.jclinane.2013.10.004 (2014).
Wang, H. et al. Incidental receipt of cardiac medications and survival outcomes among patients with stage III non-small-cell lung cancer after definitive radiotherapy. Clin. Lung Cancer 16, 128–136, doi:10.1016/j.cllc.2014.09.006 (2015).
Wang, H. M. et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. 24, 1312–1319, doi:10.1093/annonc/mds616 (2013).
Holmes, S., Griffith, E. J., Musto, G. & Minuk, G. Y. Antihypertensive medications and survival in patients with cancer: A population-based retrospective cohort study. Cancer Epidemiol. 37, 881–885, doi:10.1016/j.canep.2013.09.001 (2013).
Shah, S. M. et al. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br. J. Clin. Pharmacol. 72, 157–161, doi:10.1111/j.1365-2125.2011.03980.x (2011).
Suissa, S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 167, 492–499, doi:10.1093/aje/kwm324 (2008).
Weberpals, J., Jansen, L., Carr, P. R., Hoffmeister, M. & Brenner, H. Beta blockers and cancer prognosis - The role of immortal time bias: A systematic review and meta-analysis. Cancer. Treat. Rev. 47, 1–11, doi:10.1016/j.ctrv.2016.04.004 (2016).
van Herk-Sukel, M. P. et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur. J. Cancer 46, 395–404, doi:10.1016/j.ejca.2009.09.010 (2010).
WHO Collaborating Centre for Drug Statistics Methodology. ATC classification index with DDDs, 2011. Oslo; 2010.
Shrank, W. H., Patrick, A. R. & Brookhart, M. A. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J. Gen. Intern. Med. 26, 546–550, doi:10.1007/s11606-010-1609-1 (2011).
Rodgers, T., Leahy, D. & Rowland, M. Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J. Pharm. Sci. 94, 1237–1248, doi:10.1002/jps.20323 (2005).
Anderson, J. R., Cain, K. C. & Gelber, R. D. Analysis of survival by tumor response. J. Clin. Oncol. 1, 710–719 (1983).
Mantel, N. & Byar, D. P. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J. Am. Stat. Assoc 69, 81–86 (1974).
Mi, X., Hammill, B. G., Curtis, L. H., Lai, E. C. & Setoguchi, S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat. Med., doi:10.1002/sim.7019 (2016).
Glynn, R. J., Knight, E. L., Levin, R. & Avorn, J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology 12, 682–689 (2001).
Lund, J. L., Richardson, D. B. & Sturmer, T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr. Epidemiol. Rep 2, 221–228, doi:10.1007/s40471-015-0053-5 (2015).
Pottegard, A. & Hallas, J. New use of prescription drugs prior to a cancer diagnosis. Pharmacoepidemiol. Drug Saf., doi:10.1002/pds.4145 (2016).
Rosenbaum, P. & Rubin, D. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41–55 (1983).
Schneeweiss, S. et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am. J. Epidemiol 154, 854–864 (2001).
Austin, P. C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat 10, 150–161, doi:10.1002/pst.433 (2011).
Wang, Y. et al. Optimal caliper width for propensity score matching of three treatment groups: a Monte Carlo study. PloS one 8, e81045, doi:10.1371/journal.pone.0081045 (2013).
Austin, P. C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 33, 1057–1069, doi:10.1002/sim.6004 (2014).
Rassen, J. A., Doherty, M., Huang, W. & Schneeweiss, S. Pharmacoepidemiology Toolbox. Boston, MA. http://www.hdpharmacoepi.org.
Lin, D. Y. & Wei, L.-J. The robust inference for the Cox proportional hazards model. J. Am. Stat. Assoc 84, 1074–1078 (1989).
Faries, D., Leon, A. C., Haro, J. M. & Obenchain, R. L. Analysis of Observational Health Care Data Using SAS®, 60–81 (SAS Institute 2010).
Schoenfeld, D. A. Sample-size formula for the proportional-hazards regression model. Biometrics 39, 499–503 (1983).
Hu, M., Hu, Y., He, J. & Li, B. Prognostic Value of Basic Fibroblast Growth Factor (bFGF) in Lung Cancer: A Systematic Review with Meta-Analysis. PloS one 11, e0147374, doi:10.1371/journal.pone.0147374 (2016).
Pillai, R. N. & Owonikoko, T. K. Small cell lung cancer: therapies and targets. Semin. Oncol. 41, 133–142, doi:10.1053/j.seminoncol.2013.12.015 (2014).
Hoffmeister, M. et al. Statin use and survival after colorectal cancer: the importance of comprehensive confounder adjustment. J. Natl. Cancer Inst. 107, djv045, doi:10.1093/jnci/djv045 (2015).
Coelho, A. L. et al. Angiogenesis in NSCLC: is vessel co-option the trunk that sustains the branches? Oncotarget, doi:10.18632/oncotarget.7794 (2016).
Kabbinavar, F. et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 21, 60–65 (2003).
Sandler, A. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–2550, doi:10.1056/NEJMoa061884 (2006).
M.D. Anderson Cancer Center. Feasibility Study: Therapeutic Targeting of Stress Factors in Ovarian Cancer Patients. ClinicalTrials. gov Identifier: NCT01504126. (2012).
William Carson. Propranolol Hydrochloride in Treating Patients With Locally Recurrent or Metastatic Solid Tumors That Cannot Be Removed By Surgery. ClinicalTrials. gov Identifier: NCT02013492. (2014).
Columbia University. Study of Propranolol in Newly Diagnosed Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. ClinicalTrials. gov Identifier: NCT01847001. (2012).
Therapeutic Targeting of Stress Factors in Ovarian Cancer Patients. ClinicalTrials. gov Identifier: NCT01308944. (2010).
Kaplan Medical Center. Perioperative Administration of COX 2 Inhibitors and Beta Blockers to Women Undergoing Breast Cancer Surgery. ClinicalTrials. gov Identifier: NCT00502684 (2014).
Acknowledgements
We would like to thank Jeremy Rassen, Michael Doherty, Wei Huang and Sebastian Schneeweiss from the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston for their provision of the Pharmacoepidemiology Toolbox and the associated correspondence. Additionally we would like to thank Dr. Mahdi Fallah, Division of Preventive Oncology, National Center for Tumor Diseases (NCT), German Cancer Research Center (DKFZ), Heidelberg, Germany for his valuable suggestions on the study protocol. This work was funded in part by grants from the German Cancer Aid [No. 70110446] and the German Federal Ministry of Education and Research [No. 01ER1505A].
Author information
Authors and Affiliations
Contributions
J.W. planned the study, carried out the analyses, interpreted the data and drafted the manuscript. L.J. contributed to the study proposal, the coding of the analyses and the writing of the manuscript. H.B. and W.H. contributed to and revised the study proposal. M.W. and M.H. gave consultation on the statistical analyses and the interpretation of the data. H.B. supervised all steps of the study project. WH contributed to the interpretation of the results from a clinical context. P.V. and M.H.S. provided and validated the dataset. All authors revised drafts critically for important intellectual content, and all authors reviewed and approved the final manuscript. J.W. is the guarantor.
Corresponding author
Ethics declarations
Competing Interests
J.W., L.J., M.H., P.V. and M.W. have nothing to disclaim. H.B. reports grants from the German Cancer Aid and from German Federal Ministry of Education and Research, during the conduct of the study. M.H.S. is an employee of the PHARMO Institute for Drug Outcomes Research; this independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies. W.H. reports personal fees and non-financial support from Aqua Institute Göttingen, Aspen Europe GmbH, Diaplan, EvalueScience Ltd., Grünenthal GmbH, GSK GER/UK/Slovakia/France/Espana/Poland, and Novartis, other from Dosing GmbH, personal fees from Actelion GmbH, AstraZenica GmbH, Berlin-Chemie AG, Boehringer GmbH, Bristol-Myers Squibb GmbH, Gesundheitsamt Österreich, KWHC GmbH, MSD Sharp & Dohme GmbH, Roche, UK, and Südwestrundfunk, personal fees and other from Thieme Verlag and Daiichi Sankyo GmbH, grants and personal fees from Landesapothekerkammer Hessen/Nieders/BW, grants from BMBF (DZIF, ESTHER), EU (QUALMAT), outside the submitted work; and WEH is a member of the scientific advisory board and shareholder of Dosing GmbH, the company distributing the clinical decision support software used in this study. His wife is an employee of Dosing GmbH.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weberpals, J., Jansen, L., Haefeli, W.E. et al. Pre- and post-diagnostic β-blocker use and lung cancer survival: A population-based cohort study. Sci Rep 7, 2911 (2017). https://doi.org/10.1038/s41598-017-02913-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02913-8
This article is cited by
-
Chronic stress in solid tumor development: from mechanisms to interventions
Journal of Biomedical Science (2023)
-
Intraoperative hypotension is associated with shortened overall survival after lung cancer surgery
BMC Anesthesiology (2020)
-
β-Adrenergic Signaling in Lung Cancer: A Potential Role for Beta-Blockers
Journal of Neuroimmune Pharmacology (2020)
-
Causation between Pathway Completion and Reduced Hospital Stay in Patients with Lung Cancer: a Retrospective Cohort Study Using Propensity Score Matching
Journal of Medical Systems (2020)
-
Cardiovascular Disease and Cancer: Is There Increasing Overlap?
Current Oncology Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.