Abstract
Psychopathic traits of conduct disorder (CD) have a core callous-unemotional (CU) component and an impulsive-antisocial component. Previous task-driven fMRI studies have suggested that psychopathic traits are associated with dysfunction of several brain areas involved in different cognitive functions (e.g., empathy, reward, and response inhibition etc.), but the relationship between psychopathic traits and intrinsic brain functional architecture has not yet been explored in CD. Using a holistic brain-wide functional connectivity analysis, this study delineated the alterations in brain functional networks in patients with conduct disorder. Compared with matched healthy controls, we found decreased anti-synchronization between the fronto-parietal network (FPN) and default mode network (DMN), and increased intra-network synchronization within the frontothalamic–basal ganglia, right frontoparietal, and temporal/limbic/visual networks in CD patients. Correlation analysis showed that the weakened FPN-DMN interaction was associated with CU traits, while the heightened intra-network functional connectivity was related to impulsivity traits in CD patients. Our findings suggest that decoupling of cognitive control (FPN) with social understanding of others (DMN) is associated with the CU traits, and hyper-functions of the reward and motor inhibition systems elevate impulsiveness in CD.
Similar content being viewed by others
Introduction
Conduct disorder (CD) is a serious mental disorder of childhood and adolescence that is characterized by persistent behavioral pattern in which the basic rights of others, or rules or laws are violated1. CD patients with elevated psychopathic traits have more severe and stable antisocial behavior, and poorer response to treatment2. Such psychopathic traits have a core callous-unemotional (CU) component (lack of guilty and empathy) and an impulsive-antisocial component3. However, the neural underpinnings of these components in CD patients remain elusive.
Previous studies have revealed that conduct problems are associated with abnormal activities in brain regions implicated in a wide range of socio-cognitive functions ranging from motivation4, inhibition5, reinforcement learning3, 6,7,8,9, to empathy10. Most prominently, those focusing on empathy and emotional face recognition have consistently demonstrated a close relationship between CU traits and reduced response of amygdale, ventral medial prefrontal cortex (VmPFC) and insula to distress cues6, 11,12,13, particularly for fear and pain of others. The reduced response of those brain regions to other’s distress cues is considered to be critical for the lack of empathy in the CD patients, which may lead to reduced aversion for actions that harm others. Interestingly, a recent fMRI study found that the anti-correlation between a fronto-parietal control network (FPN) and the default mode network (DMN) during a social working memory task was negatively correlated with empathy in healthy subjects14, and another study using resting-state fMRI approach showed a significant association between the FPN-DMN anti-correlation and trait emotional intelligence15. These observations raise a possibility that the psychopathic traits in psychiatric disorders (e.g., CD) may be not only related to the dysfunction in specific brain regions, but also affected by the dynamics between core brain networks.
Unlike the CU traits, very few studies have been performed to investigate the neural correlates of impulsivity in CD patients. However, valuable insights into the neurophysiology of impulsivity have been provided by studies in healthy controls and other mental disorders involved in psychopathic impulsivity such as ADHD and substance abuse16,17,18,19, which consistently document a close relationship between impulsivity and a fronto-striatal circuit (mainly composed of VmPFC, insula and striatum) subserving reward. Dysfunction of this fronto-striatal circuit has also been observed in CD patients while attending to reinforcement learning and reversal learning tasks6. Another brain network involved in impulsivity is composed of inferior frontal gyrus, supplementary motor area (SMA), pre-motor, and parietal cortex, which is consistently observed critical for response inhibition20. Abnormal activations in the SMA, prefrontal and parietal cortices during response inhibition task have been identified in CD patients5, 21, suggesting the involvement of inhibition-related network in the neuropathology of CD impulsivity traits.
As mentioned above, task-driven fMRI researches have helped to elucidate several potential neural substrates of psychopathic traits in CD. However, constrained by limited cognitive process that one task can probe, our understanding of the neural correlates of psychopathic traits with the alteration of brain functional architecture in CD is still fragmented. Moreover, psychopathic traits, like the normal personality traits, have a complex construct which is influenced by multiple systems3, 17. It is possible that individual differences in psychopathic traits are associated with broader patterns of global information processing that may extend beyond the previously identified circumscribed brain regions by specific task-driven fMRI data. Instead, the resting-state fMRI (rsfMRI) offers us a unique opportunity to appreciate the brain-wide functional architecture. Of note, such an approach has exerted valuable insights into the neural underpinnings of several normal and abnormal personality traits such as extraversion, neuroticism and borderline personality disorder22,23,24,25,26. In this study, applying a holistic brain-wide functional connectivity analysis on resting-state fMRI data, we aimed at associating abnormalities in intrinsic brain networks of adolescents with CD with their core psychopathic traits.
We firstly hypothesized that neural modules relevant to empathy (e.g., amygdala)27 would be impaired and associated with CU traits, while the disrupted fronto-striatal circuit responsible for reward3 and the inhibition-relevant circuit would be related to impulsivity in CD patients. Secondly, given the evidence of the association between the FPN-DMN anti-correlation and empathy in healthy subjects15, we anticipated that FPN-DMN dynamic interaction might be impaired and associated with the CU traits (lack of empathy) in CD patients.
Methods
Ethical Statement
All participants and their parents gave their written informed assent and consent to participate in our study after detailed description of the risks and benefits. The study was approved by the ethics committee of the Second Xiangya Hospital, Central South University. All the subsequent research analyses were carried out in accordance with the approved guidelines.
Participants
A total of forty-two male adolescents with CD were recruited from the Second Xiangya Hospital of Central South University, Changsha, China. Forty-one matched healthy volunteers were recruited from a regular school in the same city. All subjects were right-handed according to the Edinburgh Handedness Inventory28.
Two experienced psychiatrists over 10 years diagnosed CD independently based on the Structured Clinical Interview of the Diagnostic and Statistical Manual of Mental Disorders IV, Text Revision, Axis I Disorders–Patient Edition29. Psychiatrists rated each symptom item as absent (0), subclinical (1), or clinically present (2). We did not diagnose conduct disorder (CD) based solely on information from adolescents; we also interviewed parents to obtain detailed information. Psychiatrists made final judgments on the consistency of information provided by patients and parents.
For recruitment of healthy control (HC), two investigators gave a detailed explanation of the study aim and procedure to the headmaster and teachers of the target school in person. Upon obtaining permission from the school’s administration, students who matched CD subjects’ ages, sex and years of education were selected randomly from class rosters. Volunteers who agreed to be interviewed by the psychiatrists were assessed using the SCID-I/P and Wechsler Intelligence Scale for Children–Chinese revision (C-WISC)30. Information provided by HCs was verified by their parents as needed. No HC met the criteria for CD.
Exclusion criteria for subjects in both groups were: history of attention deficit hyperactivity disorder, oppositional defiant disorder, or any other psychiatric or emotional disorder; diagnosis with any pervasive developmental or chronic neurological disorder, Tourette’s syndrome, post-traumatic stress disorder, or obsessive compulsive disorder; persistent headache or head trauma; alcohol or substance abuse in the past year; contraindication to magnetic resonance imaging; and IQ ≤ 80 according to the C-WISC. All participants with CD were treatment naïve and fulfilled the criteria for adolescent-onset CD, demonstrating at least one sign of CD after 10 years of age.
Depression and anxiety severity were rated using the Chinese version of the Center for Epidemiologic Studies Depression Scale31 and the Multidimensional Anxiety Scale for Children32, respectively. The Chinese versions of the Subjective Socioeconomic Status Scale33 and the Strength and Difficulties Questionnaire (SDQ)34 were used to assess socioeconomic status and detect internalizing and externalizing problems, respectively. The Antisocial Process Screening Device (APSD) was used to assess CU traits35, and the Barratt Impulsiveness Scale (BIS)36 was also used to assess impulsivity. These scales and questionnaires have shown enough reliability and validity in our previous studies21, 37,38,39.
fMRI data acquisition and preprocessing
Resting-state fMRI data were acquired using a Philips Gyroscan Achieva 3.0 Tesla MRI scanner in the axial location, with a gradient-echo-planar imaging sequence. Details of image acquisition and fMRI data preprocessing can be found elsewhere40,41,42 and in the Supplemental Materials (Text. S1).
Group Independent Component Analysis (ICA)
Spatial ICA was conducted with resting-state fMRI data from all 83 participants using the Informix algorithm with the Group ICA of fMRI Toolbox (GIFT) software (Medical Image Analysis Lab, University of New Mexico, Albuquerque, NM, USA; http://icatb.sourceforge.net/). The exact pipeline for ICA has been applied in our prior works43, 44 and other studies45,46,47,48,49. Based on the aggregate dataset from all participants, data dimensionality (number of components) was estimated using the minimum description length criteria tool in GIFT45, 48, 49, which suggested that 30 is the optimal number of independent components (ICs). The dimensions of the functional data were then reduced using principal component analysis45, 46, followed by an independent component estimation that produced spatial maps and time courses with the infomax algorithm46. Estimated ICs at the group level (both spatial maps and time courses) were then back-reconstructed for each participant based on principal components analysis compression and projection45, 47, 48, yielding subject-specific spatial maps and time courses for each estimated component. This specific back-reconstruction feature of the GIFT algorithm allows analysis of all participants simultaneously as part of a large ICA group matrix47. For each IC, the time courses of each component therefore represented a pattern of synchronized brain activity, whose coherency pattern across voxels was represented in the associated spatial map. To display voxels relevant to a particular IC, the intensity values in each map were converted to z values50.
Identifying resting state networks
To identify valid resting state networks (RSNs), network components were examined visually to detect obvious artifacts and correlated spatially with a priori probabilistic gray-matter, white-matter, and cerebrospinal-fluid templates using multiple regression. As suggested by Meda et al.51, components associated weakly (│β│ < 0.05) with gray matter and strongly (│β│ > 2) with white matter and cerebrospinal fluid were identified as artifacts. Statistical maps for components were created using voxel-wise one-sample t-tests, with a threshold of p < 0.05 and family-wise error (FWE) correction. Fifteen ICA components were regarded as noise, leaving 15 ICs as RSNs of interest, which were considered for further analysis (Supplemental Figure S1, Supplemental Table S1). We then related each identified network to the brain functional maps produced by Laird et al.52 using voxel-wise spatial correlation analysis to determine their likely functions (Supplemental Table S1). Finally, spatial maps of each of the 15 networks from the two groups were compared using the two-sample t-test and SPM5 software (http://www.fil.ion.ucl.ac.uk/spm5). To ensure that only highly connected regions were analyzed, we used an explicit mask created with a voxel-wise one-sample t-test (p < 0.05, FWE correction). The significance level for each network in between-group comparison was adjusted for q < 0.05 with false discovery rate (FDR) correction.
Functional Network Connectivity (FNC)
Before FNC analysis, fMRI data were band-pass filtered with a Butterworth filter with cutoff frequencies of 0.008–0.15 Hz51 (http://mialab.mrn.org/software). FNC analysis was used to examine specific temporal correlations in a nonparametric pair-wise manner, with a maximal lagged correlation approach. Time courses of all ICA networks were initially interpolated to detect finer and sub-repetition time hemodynamic differences. Then, all 15 RSNs were paired with one another to yield a total of 105 pair-wise combinations. Pair-wise correlation coefficients (representing the magnitude/extent of network connection) from all groups were extracted into SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). Resulting correlation data were transformed to Fisher’s z values and subjected to two-sample t-tests with a significance threshold at q < 0.05 with FDR correction (This FDR correction is based on all uncorrected p values from 105 two-sample t-tests).
Since recent studies53, 54 have suggested that subtle head movement is an important confounding factor for fMRI functional connectivity analysis, we also measured the head motion at each time-point which was calculated as the frame-wise displacement (FD). The mean absolute FD between HC and CD did not differ significantly (Mean ± SD_HC: 0.21 ± 0.17, Mean ± SD_CD: 0.19 ± 0.08, ns). After that, the mean absolute FD further entered into a contrast between HC and CD in a two-sample t-test to examine the group differences of intra-network or inter-network FC. The results were very similar to our prior findings without including FD as a nuisance covariate.
Behavioral correlations of intra- and inter-network FC
For patients with CD, altered intra-network and inter-network FC within/between networks with mean loading coefficients showing a main effect of group difference were first extracted, and bivariate correlation with behavioral scale scores was then examined. For multiple testing, a powerful bootstrapping method55 was applied to reduce potential spurious findings; calculations were based on 5000 bootstrapped samples using biased corrected and accelerated 95% confidence intervals (CIs). Considering that the impulsivity measured by BIS screening is a continuous variable across normal adolescents and those with behavioral problems36, we also performed behavioral correlation analysis including all participants.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Sample characteristics
Subjects’ demographic and clinical characteristics are summarized in Table 1. The two groups were well matched, with no significant difference in age, IQ, years of education, socioeconomic status, depression symptomology, or anxiety severity. SDQ total and conduct problems subscale scores, as well as APSD total and CU trait subscale scores, were significantly higher in the CD group than in the HC group. BIS total and motor and non-planning subscale scores were also significantly higher in the CD group than in the HC group, indicating that subjects with CD were more impulsive.
Altered intra-network and inter-network FC
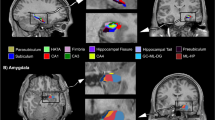
Among the 15 ICs, three brain networks showed altered intra-network FC in patients with CD (p < 0.05, FDR correction; Fig. 1, Table 2). Relative to HCs, patients showed increased FC in the frontothalamic–basal ganglia (specifically located at the left anterior insula [AI, MNI = −42, −3, −9, cluster = 59, q < 0.001 with FDR correction] and right ventral medial prefrontal cortex [VmPFC, MNI = 21, 30, 48, cluster = 23, q = 0.042 with FDR correction]), right frontoparietal (right precentral gyrus [PrCG, MNI = 60, −9, 45, cluster = 15, q = 0.046 with FDR correction]), and temporal/limbic/visual (left fusiform, MNI = −33, −51, −21, cluster = 64, q = 0.002 with FDR correction, and postcentral gyrus [PoCG, MNI = −51, −21, 45, cluster = 27, q = 0.014 with FDR correction]) networks. Moreover, after integrating all 15 ICs into one component, a more stringent FDR correction (q < 0.05) was performed based on all of the voxels in this integrated component between two groups comparison. The results were similar to the prior findings showing that except for the VmPFC, other regions (insula, precental gyrus, fusiform and postcentral gyrus) were still survived.
Altered Functional Connectivity within Brain Networks in Male Adolescents with Conduct Disorder. Compared with healthy controls (HC), patients with conduct disorder showed increased functional connectivity (FC) in the frontothalamic–basal ganglia (A), right frontoparietal (B), and temporal/limbic/visual (C) networks (q < 0.05, false discovery rate correction). All brain slices are in transverse view, with corresponding Montreal Neurological Institute slices (in millimeters).
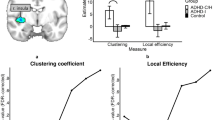
FNC analysis on the inter-FC between 15 brain networks revealed significantly increased FC only between the left fronto-parietal (FPN) and default mode network (DMN) in patients with CD compared with HCs (q < 0.05, FDR correction; Fig. 2A and B). Correlation coefficients between the FPN and DMN obtained by one-sample t-tests (p < 0.05, FWE correction) were significantly less than 0 in the HC group, suggesting a negative correlation, and greater than 0, but not significantly so, in patients with CD. We thus infer that the FPN–DMN anti-correlation was significantly reduced in patients with CD relative to HCs.
Altered Functional Connectivity between the Fronto-parietal and Default Mode Networks in Male Adolescents with Conduct Disorder. (A) Brain maps of the default mode network (DMN) and fronto-parietal network (FPN) derived from independent component analysis. (B) Anti-correlation between the DMN and FPN was reduced significantly in patients with conduct disorder (CD) compared with healthy controls (** q < 0.01, FDR correction). (C) Significant correlations between FPN-DMN inter-network FC and the callous-unemotional (CU) traits in patients with CD.
Behavioral correlations of altered intra- and inter-network FC in patients with CD
Increased FC in the left AI (belonging to the frontothalamic–basal ganglia network) was correlated positively with SDQ (r = 0.42; 95% CI, 0.11–0.69) and BIS total scores (r = 0.42; 95% CI, 0.11–0.64) (Fig. 3), as well as BIS nonplanning (r = 0.40; 95% CI, 0.06–0.66) and motor (r = 0.38; 95% CI, 0.06–0.60) subscale scores, in patients with CD (Supplemental Figure S2). Moreover, higher level of CU traits was associated with greater reduction in anti-correlation between FPN and DMN (r = 0.60; 95% CI, 0.32–0.85; Fig. 2C).
Since impulsivity is considered be a continuous variable across normal adolescents and those with behavioral problems36, correlation analysis of the BIS data from all participants were performed and showed that increased intra-network FC in the AI (frontothalamic–basal ganglia network), PrCG (right frontoparietal network), and PoCG (temporal/limbic/visual network) was correlated positively with BIS subscale scores (Supplemental Table S2).
We also did a FDR correction on the correlation findings (with a significant threshold at q < 0.05). The results with FDR correction were almost identical to the prior findings. In the patient group, the correlation of insular FC with SDQ total score still remained significant (q = 0.04), while those with BIS total score, BIS non-planning (BIS-NP) and motor (BIS-M) subscale scores all showed trends of significance (q = 0.11 for all). Across two groups, the results showed significant correlations of insular FC with BIS total score, BIS-NP score and BIS-M score (q < 0.00 for all); meanwhile, PrCG FC also showed a significant correlation with BIS-M score (q = 0.02) and a trend of significance with BIS total score (q = 0.09). For inter-network FC, the significant correlation of FPN-DMN FC with CU traits still survived after FDR correction (q < 0.001).
Discussion
To our knowledge, this study is the first to explore the relationship between intrinsic brain functional architecture and psychopathic traits in individuals with conduct problems. Our findings showed that adolescents with CD exhibited increased FC within distributed brain networks including frontothalamic–basal ganglia network, right fronto-parietal network and temporal/limbic/visual network, which are suggested to be associated with reward, response inhibition and sensory information processing respectively52. Furthermore, we also observed decreased anti-correlation between the FPN and DMN, which have been established to subserve cognitive control and internal thought, respectively56. Most interestingly, the present study revealed that the heightened couplings within brain networks were consistently associated with the impulsivity traits, while the weakened interaction between brain networks may underpin the CU traits in adolescent CD patients.
Weakened interaction between Intrinsic Networks Associated with CU Traits
Consistent with our hypothesis, the present study revealed that reduced FPN-DMN anti-correlation related to high level of CU traits (i.e., lack of empathy) in CD patients. Empathy, an ability to understand and share the mental states of others, has been reported to be associated with the DMN57,58,59. Several core regions in the FPN, such as the anterior cingulated cortex, anterior insula and right temporoparietal conjunction, have also been reported to be involved in empathy57, 60, 61. Of note, a recent resting-state fMRI study demonstrated a significant association of the FPN-DMN anti-correlation with trait emotional intelligence15. Furthermore, by applying a social working memory paradigm, Xin et al.14 found that greater reduction of the FPN-DMN anti-correlation related to higher empathic ability in healthy subjects. The evidence together suggests that the DMN and PFN activity, as well as their interaction, are all important for empathy. The current findings shed further light on the impaired FPN-DMN interaction and its contribution to the CU traits (i.e., lack of empathy) in patients with conduct problems14, 15. Our finding also concurs with a recent resting-state fMRI study showing disrupted functional integration between the DMN and attention control networks in a population with antisocial personality disorder62, suggesting that the imbalance between FPN and DMN activity may be sustained from the adolescents with CD to its severe form adult psychopathy. Future longitudinal study is strongly warranted to verify this hypothesis.
Heightened Coupling within Brain Networks Associated with Impulsivity Traits
Impulsive behaviors have been proposed to be arisen from two separate processes –increased sensation seeking in terms of pursuing rewards and deficit of motor or response inhibition20. The present study reveal that CD is involved in both neuropathological aspects of impulsivity, as evidenced by our findings of the associations of impulsivity with heightened activity in the right fronto-parietal and temporal/limbic/visual network both relevant to response inhibition, as well as the frontothalamic–basal ganglia network subserving reward processing52 in this disorder.
We found that hyper-connectivity within the frontothalamic–basal ganglia network located specifically in the VmPFC and AI, both of which are known to be critical in the computation of reward expectations3. Previously, increased VmPFC and AI activation in response to punished reversal errors studies have been documented in children with CD6 and offenders with psychopathy63. Hyper-function in these regions has been linked to impaired ability to perceive violation of reward expectations (when reward is expected but punish is received), which may lead to frustration and, in turn, give rise to impulsive decision-making and reactive aggression3. Consistent with this notion, our correlation analysis showed significant associations of insular hyper-function with impulsivity traits and conduct problems (as measured by SDQ total score) in CD patients.
Notably, these findings fit nicely with a series of findings that reward-induced decision-making structures, particularly anterior insula and VmPFC, show disruption in CD, but that this disruption is not driven by CU traits7,8,9. Furthermore, a most recent work suggests, consistent with the current findings, that impulsivity symptoms in youth with CD might be driving this dysfunction64. This work is also consistent with Rubia’s work in “clean” CD and ADHD samples4, 5, 27, suggesting that the “hot” paralimbic system regulating the movitation/reward may characterize the pathophysiology of “pure” CD. Given the consistency of these findings across studies from groups in the US, Europe and China, the functional abnormality in this reward-related frontothalamic–basal ganglia circuitry might be a potentially key treatment target for impulsivity and conduct problems for this mental disease in childhood and adolescence.
Interestingly, we also found enhanced connectivity within the right fronto-parietal network and temporal/limbic/visual network. The hyper-connectivity in the first network was specifically located at the pre-central gyrus adjacent to the frontal eye field but belonged to the supplementary motor area (SMA), and that in the second network was located at the post-central gyrus (a primary somatosensory area) and fusiform. The (pre)motor and sensorimotor cortices have been consistently observed to be involved in motor inhibition in healthy subjects20. Particularly, the increased SMA activity has been found to be correlated with poorer stop-signal task performance in healthy subjects65,66,67. In CD patients, our prior work and another study both identified dysfunction in the pre-motor and sensorimotor cortex during response inhibition task5, 21. Of note, a recent resting-state fMRI study in healthy subjects found a positive association between impulsivity and FC in the sensory/visual module17. However, our study did not observe a significant relationship between the increased fusiform FC and impulsivity. Thus, the role of the disrupted resting-state function of visual cortex in CD psychopathology still need to be examined in future studies.
What should be noted is that amygdale dysfunction was not observed in our data, which is apparently inconsistent with a previous resting-state fMRI study68. However, instead of functional connectivity with other brain regions, previous report focused on a different aspect (i.e., energy) of amygdale using the amplitude of low-frequency fluctuation (ALFF). Our findings are also different from two previous reports (n = 18) identifying uncoupling within brain networks, such as the default mode, sensory-motor, or the visual networks69, 70. Such differences may be due to the clinical heterogeneity among CD patients27. Compared to our relatively larger sample from 36 CD patients with substantial clinical profiles (6 patients were excluded for their excessive head motions, please see the supplementary Text), these previous studies using the same dataset from 18 CD adolescents reported no records on the severity of clinical symptoms and psychopathy. Thus, clinical profiles cannot be directly compared between those studies and ours. However, given the robust evidence that clinical features, such as the severity of CU traits, modify the neuropathological characteristics in CD patients10, it is possible that homogeneity of clinical features might mix the findings of resting-state brain network in this population. Moreover, unlike the previous studies, we further excluded comorbidity of ADHD, depression and anxiety, as these three had been proven to have high comorbidity rate with CD and modulate the amygdale activity27, 71.
Limitations
Several caveats of our findings should be mentioned. First, this study assessed the psychopathy across patients and healthy controls using the APSD, which so far has not been established a cutoff score for classification of a high level of psychopathy. Previous studies of adolescents have used median splits (e.g., > 11 for males, 9 for females)72, cutoff scores (such as a score of 20)6, 11, 73, or percentile rankings (e.g., the top 33%)74 to classify the level of psychopathy. If using a stringent cutoff like a score of 20, this study had only three patients with APSD scores > 20. Thus, future studies are warranted for samples with more severe psychopathy. However, it has also been suggested that using an extreme groups approach may result in relatively limited number of samples and limited variability of CU traits, raising the possibility of Type II error in the correlation analysis between neural deficits and psychopathic traits75. Given the significant differences of APSD total and CU traits scores between CD patients and HC (p < 0.003 and 0.005, respectively), it makes sense to investigate the neural correlates of psychopathy in this CD sample with large variations of CU traits scores. Second, our comparison group showed relatively higher APSD total scores comparing to previous studies from the US11, 76, 77, which may reduce the statistical significance in comparing resting-state networks between two groups. Since we have carefully excluded subjects with any psychiatric or neurological disease in HC group by two experienced psychiatrists, future studies, particularly for Chinese sample, should keep in mind on this issue to verify whether the average level of APSD scores of Chinese adolescents is higher relative to western world. Third, we did not record the illness duration of the CD patients, which might be a potential confounding factor. Forth, by using a FDR correction on our correlation findings, it is interesting to note that the correlation of insular FC with BIS scores survived in two groups, but showed a trend of significance in the patient group. A possible reason is that our sample with 36 patients is not large enough to detect the statistic significance of this correlation at a stringent threshold. Future studies with larger samples are needed to verify the associations of resting-state insular dysfunction with impulsivity traits. Finally, as resting-state network did not explicitly engage any cognitive tasks, further examination of FPN-DMN dynamic interaction in CD patients during tasks such as decision making in social context may reveal dysfunction in more specific dimension of the interplay between the executive function and the social understanding to others.
In summary, while current theories of biological substrates of psychopathic traits in CD are predominantly arisen from task-driven fMRI approaches focusing on functional alterations in specific brain regions, the present data raise the possibility that more global network dysfunctions may also be underpin the core psychopathic traits in this disorder. Critically, our study provides initial evidence that the FPN-DMN anti-correlation is associated with the CU traits. Our observations underscore the importance of spontaneous dynamic among large-scale networks in the mechanism of psychopathic traits in CD, and raise the question whether restoring this diminished competition between core intrinsic networks should be a novel treatment target for focused training, neuromodulation or pharmacotherapy approaches that purport to overcome CU traits – one of the most persistent challenges in treating conduct problems.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing (2013).
Frick, P. J., Ray, J. V., Thornton, L. C. & Kahn, R. E. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin 140, 1–57 (2014).
Blair, R. J. R. The neurobiology of psychopathic traits in youths. Nature reviews. Neuroscience 14, 786–799 (2013).
Rubia, K. et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry 166, 83–94 (2009).
Rubia, K. et al. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry 165, 889–897 (2008).
Finger, E. C. et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of general psychiatry 65, 586–594 (2008).
White, S. F. et al. Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. Journal of the American Academy of Child & Adolescent Psychiatry 53, 579–588.e579 (2014).
White, S. F. et al. Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am J Psychiatry 170, 315–323 (2013).
White, S. F. et al. Neural Correlates of the Propensity for Retaliatory Behavior in Youths With Disruptive Behavior Disorders. Am J Psychiatry 173, 282–290 (2016).
Viding, E. et al. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am J Psychiatry 169, 1109–1116 (2012).
White, S. F. et al. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am J Psychiatry 169, 750–758 (2012).
Jones, A. P., Laurens, K. R., Herba, C. M., Barker, G. J. & Viding, E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry 166, 95–102 (2009).
Lockwood, P. L. et al. Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Current biology: CB 23, 901–905 (2013).
Xin, F. & Lei, X. Competition between frontoparietal control and default networks supports social working memory and empathy. Social cognitive and affective neuroscience 10, 1144–1152 (2015).
Takeuchi, H. et al. Resting state functional connectivity associated with trait emotional intelligence. NeuroImage 83, 318–328 (2013).
Bari, A. & Robbins, T. W. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology 108, 44–79 (2013).
Davis, F. C. et al. Impulsivity and the modular organization of resting-state neural networks. Cerebral cortex 23, 1444 (2013).
Hart, G. Impulsivity Resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli,” Psychopharmacology. International Journal of Solids & Structures 47, 1424–1434 (2010).
Hoogman, M. et al. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. American Journal of Psychiatry 168, 1099–1106 (2011).
Bari, A. & Robbins, T. W. Inhibition and impulsivity Behavioral and neural basis of response control. Progress in Neurobiology 108, 44–79 (2013).
Zhang, J. et al. Impaired Frontal-Basal Ganglia Connectivity in Male Adolescents with Conduct Disorder. PLoS One 10, e0145011 (2015).
Adelstein, J. S. et al. Personality Is Reflected in the Brain’s Intrinsic Functional Architecture. Plos One 6, e27633 (2011).
Aghajani, M. et al. Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cognitive, Affective, & Behavioral Neuroscience 14, 836–848 (2014).
Krauseutz, A. et al. Amygdala and anterior cingulate resting-state functional connectivity in borderline personality disorder patients with a history of interpersonal trauma. Psychological medicine 44, 1–13 (2014).
Lei, X., Zhao, Z. & Chen, H. Extraversion is encoded by scale-free dynamics of default mode network. NeuroImage 74, 52–57 (2013).
Wei, L. et al. Specific frequency bands of amplitude low-frequency oscillation encodes personality. Human Brain Mapping 35, 331–339 (2014).
Rubia, K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biological psychiatry 69, e69–87 (2011).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
First, M. B. Structured clinical interview for DSM-IV-TR Axis I disorders: patient edition (Biometrics Research Department, Columbia University, 2005).
Gong Yaoxian, C. T. Wechsler Intelligence Scale for Children, Chinese Revision (C-WISC) (Hunan Maps Press, Changsha, 1993).
Radloff, L. S. The CES-D scale a self-report depression scale for research in the general population. App Psych Meas 1, 385–401 (1977).
Yao, S. et al. Reliability and validity of the Chinese version of the Multidimensional Anxiety Scale for Children among Chinese secondary school students. Child psychiatry and human development 38, 1–16 (2007).
Hu, M., Cai, W. M., Zhu, L. & Yao, X. S. Development of Subjective Socioeconomic Status Scale for Chinese adolecents. Chin J Clin Psychol 20, 155–161 (2012).
Yao, S. et al. Measuring adolescent psychopathology: Psychometric properties of the self-report Strengths and Difficulties Questionnaire in a sample of Chinese adolescents. J Adolescent Health 45, 55–62 (2009).
Vitacco, M. J., Rogers, R. & Neumann, C. S. The antisocial process screening device an examination of its construct and criterion-related validity. Assessment 10, 143–150 (2003).
Yao, S. et al. An examination of the psychometric properties of the Chinese version of the Barratt Impulsiveness Scale, 11th version in a sample of Chinese adolescents. Percept Mot Skills 104, 1169–1182 (2007).
Jiang, Y. et al. Abnormalities of cortical structures in adolescent-onset conduct disorder. Psychological medicine 45, 3467–3479 (2015).
Jiang, Y. et al. Disrupted Topological Patterns of Large-Scale Network in Conduct Disorder. Scientific reports 6, 37053 (2016).
Zhang, J. et al. Increased structural connectivity in corpus callosum in adolescent males with conduct disorder. J Am Acad Child Adolesc Psychiatry 53, 466–475 e461 (2014).
Liu, C. et al. Abnormally increased and incoherent resting-state activity is shared between patients with schizophrenia and their unaffected siblings. Schizophrenia research 171, 158–165 (2016).
Pu, W. et al. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophrenia research 141, 15–21 (2012).
Pu, W. et al. Failed cooperative, but not competitive, interaction between large-scale brain networks impairs working memory in schizophrenia. Psychological medicine 46, 1211–1224 (2016).
Zhu, X. et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological psychiatry 71, 611–617 (2012).
Zhou, L. et al. Inefficient DMN Suppression in Schizophrenia Patients with Impaired Cognitive Function but not Patients with Preserved Cognitive Function. Scientific reports 6, 21657 (2016).
Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. A method for making group inferences from functional MRI data using independent component analysis. Human brain mapping 14, 140–151 (2001).
Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Human brain mapping 13, 43–53 (2001).
Erhardt, E. B. et al. Comparison of Multi-Subject ICA Methods for Analysis of fMRI Data. Human brain mapping 32, 2075–2095 (2011).
Khadka, S. et al. Is Aberrant Functional Connectivity A Psychosis Endophenotype? A Resting State Functional Magnetic Resonance Imaging Study. Biological psychiatry 74, 458–466 (2013).
Meda, S. A. et al. Differences in Resting-State Functional Magnetic Resonance Imaging Functional Network Connectivity Between Schizophrenia and Psychotic Bipolar Probands and Their Unaffected First-Degree Relatives. Biological psychiatry 71, 881–889 (2012).
Stevens, M. C., Kiehl, K. A., Pearlson, G. D. & Calhoun, V. D. Brain Network Dynamics During Error Commission. Human brain mapping 30, 24–37 (2009).
Meda, S. A. et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biological psychiatry 71, 881–889 (2012).
Laird, A. R. et al. Behavioral interpretations of intrinsic connectivity networks. Journal of cognitive neuroscience 23, 4022–4037 (2011).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Van Dijk, K. R., Sabuncu, M. R. & Buckner, R. L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438 (2012).
Wasserman, S. & Bockenholt, U. Bootstrapping: applications to psychophysiology. Psychophysiology 26, 208–221 (1989).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America 102, 9673–9678 (2005).
Bernhardt, B. C. & Singer, T. The neural basis of empathy. Annual review of neuroscience 35, 1–23 (2012).
Schnell, K., Bluschke, S., Konradt, B. & Walter, H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. NeuroImage 54, 1743–1754 (2011).
Spreng, R. N., Mar, R. A. & Kim, A. S. N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of cognitive neuroscience 21, 489–510 (2009).
Corbetta, M., Patel, G. & Shulman, G. L. The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324 (2008).
Fan, Y., Duncan, N. W., de Greck, M. & Northoff, G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and biobehavioral reviews 35, 903–911 (2011).
Tang, Y., Jiang, W., Liao, J., Wang, W. & Luo, A. Identifying Individuals with Antisocial Personality Disorder Using Resting-State fMRI. Plos One 9 (2014).
Gregory, S. et al. Punishment and psychopathy: a case-control functional MRI investigation of reinforcement learning in violent antisocial personality disordered men. Lancet Psychiat 2, 153–160 (2015).
Hwang, S. et al. Dual neurocircuitry dysfunctions in disruptive behavior disorders: emotional responding and response inhibition. Psychological medicine 46, 1485–1496 (2016).
Aron, A. R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological psychiatry 69, 55–68 (2011).
Chao, H. H. A., Luo, X., Chang, J. L. K. & Li, C. S. R. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—An intra-subject analysis. BMC neuroscience 10, 1–10 (2009).
Congdon, E. et al. Engagement of large-scale networks is related to individual differences in inhibitory control. NeuroImage 53, 653–663 (2010).
Zhou, J., Yao, N., Fairchild, G., Zhang, Y. & Wang, X. Altered hemodynamic activity in conduct disorder: a resting-state FMRI investigation. PLoS One 10, e0122750 (2015).
Zhou, J. et al. Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging and Behavior 1–9 (2015).
Lu, F. M. et al. Functional Connectivity Estimated from Resting-State fMRI Reveals Selective Alterations in Male Adolescents with Pure Conduct Disorder. PLoS One 10, e0145668 (2015).
Loeber, R., Burke, J. D., Lahey, B. B., Winters, A. & Zera, M. Oppositional Defiant and Conduct Disorder: A Review of the Past 10 Years, Part I. Journal of the American Academy of Child & Adolescent Psychiatry 39, 1468–1484 (2000).
Vitale, J. E. et al. Deficient behavioral inhibition and anomalous selective attention in a community sample of adolescents with psychopathic traits and low-anxiety traits. J Abnorm Child Psychol 33, 461–470 (2005).
Marsh, A. A. et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 165, 712–720 (2008).
Murrie, D. C. & Cornell, D. G. Psychopathy screening of incarcerated juveniles: a comparison of measures. Psychological assessment 14, 390–396 (2002).
White, S. F. et al. Dysfunctional representation of expected value is associated with reinforcement-based decision-making deficits in adolescents with conduct problems. Journal of Child Psychology and Psychiatry 57 (2016).
Blair, R. J., Budhani, S., Colledge, E. & Scott, S. Deafness to fear in boys with psychopathic tendencies. Journal of child psychology and psychiatry, and allied disciplines 46, 327–336 (2005).
White, S. F. et al. Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Dev Psychopath 24, 1105–1116 (2012).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81401125 to W.D.P., and 81471384 to S.Q.Y., 11471081 and 11101429 to Q.L.) and Specialized Research Fund for the Doctoral Program of Higher Education (20130162110043 to S.Q.Y.). Q.L. was also supported by the International (Regional) Collaborative and Exchange Program of National Natural Science (No. 71661167002), and the Key Project of Shanghai Science & Technology Innovation Plan (No. 15JC1400101).
Author information
Authors and Affiliations
Contributions
W.D.P., and S.Q.Y. designed the study and wrote the protocol; Y.L.J., Y.D.G., Q.S.M. collected the imaging data and clinical information; W.D.P. and Q.L. managed the reference search and analysis; W.D.P. undertook the statistical analysis, and W.D.P. wrote the first draft of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pu, W., Luo, Q., Jiang, Y. et al. Alterations of Brain Functional Architecture Associated with Psychopathic Traits in Male Adolescents with Conduct Disorder. Sci Rep 7, 11349 (2017). https://doi.org/10.1038/s41598-017-11775-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11775-z
This article is cited by
-
Associations of bullying perpetration and peer victimization subtypes with preadolescent’s suicidality, non-suicidal self-injury, neurocognition, and brain development
BMC Medicine (2023)
-
The transition trajectories of self-injurious thoughts and behaviours among children from a biopsychosocial perspective
Nature Mental Health (2023)
-
Objective self-awareness theory and violence: A brain network perspective
Neuroscience and Behavioral Physiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.