Abstract
Status of Fok I VDR polymorphism along with vitamin D, Vitamin D receptor (VDR), and cathelicidin levels in Tuberculosis (TB) patients compared to household contacts and implication of these findings in susceptibility to TB is not known. 150 active TB patients, 150 household contacts and 150 healthy controls were recruited from North Indian population. Fok1 VDR polymorphism was studied by polymerase chain reaction- restriction fragment length polymorphism (PCR-RFLP).VDR mRNA and protein levels were studied using quantitative real time PCR (q rt PCR) and enzyme linked immunosorbent assay (ELISA) respectively. Cathelicidin and Vitamin D levels were measured using ELISA and chemiluminescence immunoassay (CLIA) respectively. Significant association was found between Fok1 polymorphism and susceptibility to TB (P < 0.0005). VDR mRNA, VDR protein and vitamin D levels were significantly lower in active TB group when compared to household contacts and healthy controls (P < 0.0001, 0.0001 and 0.0005 respectively). Cathelicidin levels were higher in active TB patients compared to other groups (P < 0.0001). Expression of VDR and cathelicidin was significantly higher among ‘FF’ genotypes of VDR (more active form of VDR) compared to ‘ff’ genotype (less active form of VDR). ‘f’ allele was associated with increased susceptibility to TB. Higher frequency of ‘F’ allele, increased VDR expression along with increased vitamin D levels in household contacts compared to active TB group might be responsible for protection against active TB.
Similar content being viewed by others
Introduction
Tuberculosis (TB) remains one of the most important causes of death from an infectious agent, with a latest World Health Organization (WHO) figures indicating an estimating 10.4 million new incident cases globally in 2017, out of which 26% incident cases are from India1. The main etiologic agent of tuberculosis is an intracellular slightly gram positive bacteria, Mycobacterium tuberculosis (M.TB.). This intracellular bacterium can evade the host immune system and can remain in the macrophages of the host in dormant stage until the immunity is suppressed. The question arises, why the infection remains in latent phase in some individuals and active in others? What are the host factors that play role in activation of the disease? It is seen that some household contacts of tuberculosis patient may or may not develop tuberculosis even after the long term exposure to M.TB.
There are various environmental and genetic factors which are responsible for susceptibility to TB. Two such factors are vitamin D and its nuclear vitamin D receptor (VDR). Vitamin D acts as an immunomodulatory molecule and plays a role in the innate immune response in individuals to certain infectious agents, M.TB2. Binding of the active form of Vitamin D to its nuclear receptor VDR leads to formation of an active transcriptional complex, which increases expression of a number of proteins. One such protein is cathelicidin (LL37) which is involved in killing of M.TB.
Upon M.TB. infection monocyte toll-like receptors (TLR1–TLR2) gets activated which results in the upregulation of VDR and the vitamin-D-activating enzyme 25-hydroxyvitamin D-1α-hydroxylase. 25-hydroxyvitamin D is bound to serum vitamin-D-binding protein while in circulation. It is converted to its active form 1, 25-dihydroxyvitamin D by mitochondrial 25-hydroxyvitamin D-1α-hydroxylase in macrophages and then binds to VDR and acts as a transcription factor leading to the induction of cathelicidin (LL37) expression and promotion of autophagy. Antimycobacterial action of cathelicidin and autophagy may then combine to enhance bacterial killing3,4.
Cathelicidins are family of proteins consisting of C- terminal cationic anti-microbial peptide (CAMP) domain that is activated by cleavage from N- terminal cathelin portion of propeptide. These proteins are stored in the granules of neutrophils from which it can be released at sites of microbial infection5. Various white blood cells (WBC) populations also express this peptide6. It has been shown that exogenous addition of cathelicidin or endogenous overexpression of cathelicidin in macrophages significantly reduces the intracellular survival of M.TB. relative to control cells7. However the role of vitamin D and its receptor in controlling growth of M.TB. in vivo is a complex process and is not fully understood.
There is much variability among the individuals in response to M.TB. infections, but it is not known why certain people develop TB when infected with M.TB. while others remain latently infected. Genetic susceptibility has been suggested as one of the most important explanations for individual risk for TB. Studies have suggested a role for host genetics in TB susceptibility with respect to VDR gene polymorphism8,9,10. The Vitamin D receptor (VDR) gene is located on the long (q) arm of chromosome 12 at position 13.11. After binding to its ligand, vitamin D, VDR forms a heterodimer with retinoid X receptor. This heterodimer binds to vitamin D response elements (VDREs) in the promoter region of vitamin D3 regulated genes, enabling the interaction of regulatory proteins thereby mediating transcription. Various VDR polymorphisms have been observed in association with TB, particularly in the Fok1 SNP in exon2, Bsm1 in intron 8 and Taq 1 in intron 9 but with inconclusive findings9,10,11,12,13. As the function of vitamin D at nuclear level is carried along with VDR, it is pertinent to study VDR polymorphism. The Fok1 polymorphism is one of the functional and important polymorphism of VDR gene.
Fok I polymorphism results in different translation initiation sites due to thymine (T) to cytosine (C) substitution in the first translation initiation codon ATG which generates long and short forms of VDR. In the VDR ‘ff’ variant, initiation of translation occurs at the first ATG site, giving rise to a full length VDR protein comprised of 427 amino acids. In the VDR ‘FF’ variant, translation begins at the second ATG site due to the substitution at first site, resulting in a truncated protein with 424 amino acids. It has also been reported that ‘FF’, the shorter form of VDR protein is the more active form than its full length protein, ‘ff’14,15. The Fok1 polymorphism thus may affect function of VDR resulting in altered efficiency of binding to vitamin D, which in turn may affect production of downstream molecules such as cathelicidin. Hence this polymorphism could be one of the factors influencing increased susceptibility to TB infection.
Studies so far have correlated low levels of vitamin D and VDR polymorphism independently with occurrence of TB. The association of functional VDR polymorphism (Fok1) on expression of VDR and cathelicidin levels in TB patients is still unknown. Also the levels of VDR and cathelicidin in household contacts of TB patients have not been studied. This study aimed to explore the role of vitamin D, VDR, its polymorphism and their association on production of cathelicidin in vivo in TB patients and their household contacts at levels of vitamin D prevalent in north Indian population.
Results
Study population
A total of 150 newly diagnosed active TB patients and 150 household contacts of active TB patients were recruited. 150 healthy individuals with no known history of TB were recruited as controls. For our study we included only pulmonary tuberculosis (only lung parenchyma involved) based on inclusion and exclusion criteria (discussed in methodology section). A case of pulmonary TB phenotype is described as: a clinically diagnosed case of TB affecting the lungs, having symptoms of fever or cough and sputum smear that showed acid-fast bacilli or culture positive for M.TB. or Gene Xpert positive. We excluded all patients with concurrent pulmonary–extrapulmonary disease. Therefore only one clinical phenotype as described above was studied in the present study. The mean age of active TB patients was 39.4 ± 8.1 years, household contacts was 35.4 ± 6.6 years and healthy controls was 32.1 ± 4.1 years. The demographic and clinical characteristics of study participants are shown in Table 1.
Significant association of Fok 1 VDR polymorphism with susceptibility to TB
The Fok 1 VDR polymorphic variant obtained by using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) using Fok1 restriction enzyme is shown in Fig. 1. The genotypic distribution was in Hardy-Weinberg equilibrium. The frequencies of Fok I genotypes at VDR locus showed a significant difference in the distribution of genotypes. ‘ff’ homozygous genotype was observed in 24.6% of the active TB patients, 9.3% of household contacts and 8.6% of healthy controls. The ‘Ff’ heterozygous genotype was observed in 38.6% of patients as compared to 36.6% of household contacts and 34.0% of controls. ‘FF’ homozygous genotype was present in 36.6% of patients as compared to 54% of household contacts and 57.3% of controls. The frequency of ‘ff’ (long form of VDR) genotype was higher in patient group and that of ‘FF’ genotype was higher in control group and the differences were statistically significant (χ2 value of 25.22, df of 4 and p value = 0.0001). The frequency of ‘F’ allele was found to be 56% in active patients and 72.3% in household contacts as compared to 74.3% in healthy controls. The frequency of ‘f’ allele was found to be 44% in active patients, 27.7% in household contacts and 25.7% in healthy controls. The difference was found to be significant suggestive of an association of Fok1 VDR polymorphism with susceptibility to TB (Tables 2 and 3). Out of 150 patients, 118 were found to be sputum positive with variable sputum grade, 25 being +1, 81 being +2 and 12 being + 3. The frequency of genotype in different sputum grades (+1, +2 and + 3) were also analysed and found to be associative with smear grades (Table 4).
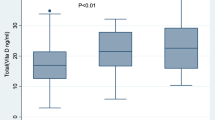
Low levels of vitamin D and its associated molecules active TB patients. 1a and 1b show the expression of VDR at mRNA and protein level respectively and (c,d) show the serum levels of cathelicidin and vitamin D in active TB patients, Household contacts and Controls. Data is represented as median ± standard deviation and each point represents individual sample value. Mann-Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisk (**) indicate a p-value < 0.01, three asterisk (***) indicate a p-value < 0.001 and four asterisk (****) indicate a p-value < 0.0001. Active TB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB.
Lower mRNA expression of VDR in active TB patients as compared to household contacts and healthy controls
VDR mRNA expression in WBCs of active TB patients and household contacts was measured by quantitative real-time PCR (q rt PCR) and was compared to healthy controls. Comparison of VDR expression was made by determining the threshold cycle (Ct) values and was then normalized with Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). We have observed that active TB patients had significantly lower expression of VDR mRNAs compared to household contacts and healthy controls (P < 0.001 and 0.0001 respectively) (Fig. 1a).
Lower serum levels of VDR and higher levels of cathelicidin (LL37) in active TB patients compared to household contacts and healthy controls
The serum levels of vitamin D receptor and cathelicidin in active TB patients, household contacts and control group were measured by sandwich ELISA. A significant difference in the serum levels of vitamin D receptor and cathelicidin was observed among all the three groups with p < 0.0001. The levels of VDR were lower in patient groups (5.13 ± 2.6 ng/ml) as compared to household contacts (6.69 ± 3.17 ng/ml) and healthy controls (7.30 ± 4.15 ng/ml) with p < 0.001 and 0.0001 respectively. The levels of VDR in household contacts and healthy controls were comparable. The serum levels of cathelicidin was significantly higher in active TB group (10.36 ± 5.62 ng/ml) as compared to household contacts (8.48 ± 5.45 ng/ml) and healthy controls (5.99 ± 4.44 ng/ml) with p < 0.01 and 0.0001 respectively. Unlike VDR, a significant difference was observed in the cathelicidin levels between household contacts and healthy controls (p < 0.01) (Fig. 1b,c).
Lower serum levels of Vitamin D in active TB patients compared to household contacts and healthy controls
Serum vitamin D levels varied among all the groups with lowest levels seen in active TB patient group (13.28 ± 5.0 ng/ml) as compared to household contacts (21.62 ± 5.99 ng/ml), and healthy controls (23.31 ± 8.2 ng/ml). The difference was statistically significant (p < 0.0001). The levels were comparable between household contacts and healthy controls (Fig. 1d).
Association of genotype with VDR and cathelicidin expression
A comparison of the expression of VDR among different genotypes of VDR irrespective of the study groups showed that samples with ‘FF’ genotype (more active form of VDR) have higher expression of VDR at both mRNA and serum protein level as compared to ‘ff’ genotype (less active form of VDR) (p < 0.0001 and 0.0001 respectively) (Fig. 2a, 2b). The serum levels of cathelicidin were found to be significantly higher in ‘FF’ genotype as compared to ‘Ff’ and ‘ff’ genotype with (p < 0.01) (Fig. 2c).
Association of genotype with VDR and cathelicidin levels irrespective of study groups: (a,b) shows the expression of VDR at mRNA and protein levels respectively with respect to different genotypes. (c) shows the level of cathelicidin with respect to different genotypes. Mann-Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisk (**) indicate a p-value < 0.01, three asterisk (***) indicate a p-value < 0.001 and four asterisk (****) indicate a p-value < 0.0001. Active TB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB.
Comparison of the expression levels of both VDR and cathelicidin among different genotypes within each of the groups showed a significant difference between ‘FF’ and ‘ff’ genotypes, with individuals having ‘FF’ genotypes have higher levels of VDR and cathelicidin as compared to individuals having ‘ff’ genotype (Figs 3–5).
Association of genotype with mRNA expression of VDR and cathelicidin levels in different study groups: (a–c) shows the expression of VDR mRNA in active TB patients, household contacts and healthy controls respectively with respect to different genotypes. Mann-Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisk (**) indicate a p-value < 0.01, three asterisk (***) indicate a p-value < 0.001 and four asterisk (****) indicate a p-value < 0.0001. Active TB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB.
Association of genotype with serum VDR levels in different study groups: (a–c) shows the level of VDR protein in active TB patients, household contacts and healthy controls respectively with respect to different genotypes. Mann-Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisk (**) indicate a p-value < 0.01, three asterisk (***) indicate a p-value < 0.001 and four asterisk (****) indicate a p-value < 0.0001. Active TB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB.
Association of genotype with serum cathelicidin levels in different study groups: (a–c) shows the level of cathelicidin in active TB patients, household contacts and healthy controls respectively with respect to different genotypes. Mann-Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisk (**) indicate a p-value < 0.01, three asterisk (***) indicate a p-value < 0.001 and four asterisk (****) indicate a p-value < 0.0001. Active TB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB.
Discussion
Tuberculosis burden could be reduced with decreasing incidence of new infection and effective therapy of newly diagnosed cases. Household contacts are at higher risk of developing latent or active TB or complete elimination of TB depending upon the host immune response. First line of defense against TB is innate immunity comprises of effector functions of innate immune cells. Therefore host factors affecting innate immunity could affect outcome of the infection. So, with this background the research question was to study host factors affecting innate immunity in pulmonary TB. Vitamin D is an immunomodulatory molecule; along with VDR it induces expression of various proteins like cathelicidin which is an important antimicrobial agent for intracellular pathogens. However VDR polymorphism could affect synthesis and functionality of VDR protein, affecting cathelicidin. Therefore this study intended to assess vitamin D levels along with effect of VDR polymorphism on disease outcome.
The present study showed significant association of Fok1 SNP (f allele) with the susceptibility to TB. The findings are in concordance with one of the study in Chinese Han population showing association of ‘ff’ genotype with susceptibility to TB9. Similar findings were observed in a north Indian population showing significant association of Fok1 VDR polymorphism with leprosy16. However, in other studies from Egypt and India, no significant association was observed for VDR Fok1 polymorphism with susceptibility to TB10,17. Interestingly, in one of the study in south Indian population, the wild type genotype (‘FF’) was found to be associated with susceptibility to TB11, whereas in the present study, ‘ff’ genotype (homozygous mutant) was found to be associated with susceptibility to TB. In a meta analysis for VDR Fok1 polymorphism in TB, several other VDR polymorphism like Bsm1, Taq1, Apa1 and Fok1 has also been studied showing Fok1 association with susceptibility to TB in east Asian population18. The diversity of genetic background in different populations may contribute to variable genotype frequencies of VDR polymorphisms and its inconsistent association with TB development.
Further, the expression of VDR at mRNA levels was found to be significantly lower in WBCs of active TB patients as compared to household contacts and healthy controls. In an earlier study conducted in pulmonary TB patients, expression of VDR mRNA was found to be significantly higher in M.TB. stimulated macrophage cultures compared to unstimulated cultures19. The results were in accordance with another study in which lower expression of VDR was observed in leprosy patients as compared to controls20. Based on observations made in present study, it was hypothesized that M.TB. may alter the expression of some of the genes like VDR in order to evade the host immune system, however the exact mechanism needs to be studied. Another study based on microarray has shown a 3.3 fold downregulation in VDR expression in cells upon infection with M.TB.21. Similar to VDR mRNA expression, VDR protein levels were found to be lower in active TB group as compared to household contacts and healthy controls. Therefore lower expression of VDR in the active TB patient group in the present study may be due to an attempt by the bacteria to downregulate the expression of VDR for its own advantage, so that cathelicidin levels would be hampered and the bacteria can survive in the host. This is the first study wherein VDR expression in household contacts was assessed and found to have higher expression of VDR as compared to TB patients. This might be due to the sufficient vitamin D levels present in the household contacts. However, the exact mechanism behind the same needs to be studied further.
As VDR is regulated by its ligand, vitamin D, its levels were measured in all the groups. A lower vitamin D level in active TB patients as compared to both household contacts and normal healthy controls was observed. These findings are in concordance with studies showing lower serum levels of vitamin D in tuberculosis patients22,23. Low levels of vitamin D in active TB patient group may be one of the factors leading to decreased expression of VDR and hence increased susceptibility to TB.
The serum levels of antimicrobial peptide, cathelicidin was also assessed and found to be higher in active TB patients as compared to both household contacts and healthy controls. Present findings are in accordance with one of the study in which active TB patient group had higher levels of cathelicidin as compared to healthy controls24,25. Similarly, increase in cathelicidin level in active disease is also documented in an in vitro model where it was shown that multiplication of bacteria stimulated TLR by a pathogen-derived ligand, which acted as a trigger for upregulation of cathelicidin to exert its anti-microbial activity3. Even though vitamin D insufficiency was observed in active TB patients, cathelicidin levels were found to be higher. Therefore, localized or intracellular cathelicidin concentrations needs to be evaluated for better understanding of interaction between vitamin D status and localized LL-37 responses within immune cells and at localized immunologic barrier sites. This finding can be supported by a study where healthy volunteers who received vitamin D supplementation showed correlation between serum 25(OH)D concentrations and cathelicidin expression in primary ex vivo cultures of human monocytes, whereas systemic concentrations of cathelicidin did not correlate with serum vitamin D concentrations26. In the present study, higher levels of cathelicidin was observed in household contacts compared to healthy controls. Another study has shown that cathelicidin antimicrobial peptide (CAMP) gene expression was significantly augmented in progressive TB, whereas in latent TB it was similar to the control group27. Based upon above findings, it could be hypothesized that household contacts of active TB patients might have some M.TB. infection which could be the reason for increased level of cathelicidin compared to healthy controls, sufficient to curtail overwhelming multiplication, though lower than active TB patients.
Levels of VDR and cathelicidin in active TB patients based on their sputum grade was also compared. No significant difference in the levels of either VDR and cathelicidin in different sputum AFB grades of patients (data not shown) was found. However, the same needs to be validated in larger sample size as in our study, out of 150 patients, we had 118 sputum smear positive patients, out of which only 12 were found to be in + 3 grade.
Till date, no study has been conducted to assess the association of functional Fok1 VDR genotypes with its downstream molecules like vitamin D receptor and cathelicidin. Here in for the first time it was observed that samples with ‘FF’ genotype (more active form of VDR) have higher expression of VDR compared to ‘ff’ (less active form of VDR) genotype, suggesting the association of ‘FF’ VDR genotype with higher expression of VDR might have protective role against TB. However results need to be validated in a larger sample population.
Since one of the effector molecule in vitamin D pathway is cathelicidin, which is responsible for killing of the bacteria, association between Fok1 genotypes and cathelicidin levels were assessed. Higher levels of cathelicidin were found to be associated with ‘FF’ (active form of VDR) genotype suggesting their plausibly combined protective role against the disease. Subgroup analysis for levels of VDR and cathelicidin among different grades of sputum AFB positive patients with respect to different genotypes was done to assess any association of genotype and levels of VDR and cathelicidin with respect to disease severity. However, no significant association was observed suggesting that ‘ff’ genotype is associated with lower levels of VDR and cathelicidin but it did not correlated with disease severity. However, apart from the association of VDR genotypes with VDR protein and cathelicidin, several other environmental and host factors might also play an important role in susceptibility to TB.
To conclude, the ‘ff’ genotype of VDR Fok1 polymorphism was found to be significantly associated with susceptibility to TB. High expression levels of VDR and vitamin D in household contacts of TB patients are implicative of the protective role of vitamin D against activation of TB and should be studied further to be considered as a preventive strategy against development of active TB. Also, cathelicidin can be used as biomarker for diagnosing TB amongst household contacts, however, the same needs to be validated in larger sample size. The limitation of the study is that household contacts have not been followed up for development of active disease. Also, localized levels of cathelicidin and VDR needs to be assessed in BAL (bronchoalveolar lavage) samples of pulmonary TB patients to get a more mechanistic interpretation. Further studies can be done to follow up this high risk group for assessing the change in the vitamin D related host factors with development of active disease to use in preventive strategy.
Materials and Methods
Study Participants
The present cross sectional study was carried out in the Department of Biochemistry at All India Institute of medical Sciences (AIIMS). Active TB patients (Newly diagnosed laboratory confirmed cases of pulmonary tuberculosis) who had not received more than 2 weeks of antitubercular treatment (ATT) were recruited from directly observed treatment (DOTS) Centre, AIIMS, New Delhi, India based on inclusion and exclusion criteria. A case of pulmonary TB was defined as a clinically diagnosed case of TB affecting the lungs, having symptoms of fever or cough and sputum smear that showed acid-fast bacilli or culture positive for M.TB. or Gene Xpert. Exclusion criteria included patients with extra-pulmonary and drug resistant tuberculosis, HIV, diabetes, hypertension, significant organ dysfunction of heart, liver and kidney, pregnant or lactating women. Additional exclusion criteria were known hypersensitivity to ATT, seizure disorder, abnormal hematologic function, inflammation like autoimmune disease, atopic dermatitis or any other uncontrolled concurrent illness. Patients with prior anti-microbial drug treatment of TB for longer than two weeks and any history of alcohol and drug abuse were also excluded.
Household contacts of TB patients aged between 18–60 years who had spent atleast 6 hours per day for atleast 2 months prior with the TB patient before start of ATT with no clinical evidence of TB were recruited. The exclusion criteria were same as for the active TB patients. Mantoux test was done in household contacts. Mantoux negative individuals were directly recruited under household contacts group. Mantoux positive individuals were followed up by sputum test or chest X-ray. Individuals negative for these tests were recruited under household contacts. Individuals with positive test were further investigated for active disease. Individuals with altered clinical parameters like Hemogram, liver function test, kidney function test, blood glucose, etc and those who were taking vitamin D supplementation were excluded from the study. Normal healthy controls were recruited from the general population with no history of known contacts with TB patients and no clinical signs and symptoms of tuberculosis and no history of any other chronic disorder or inflammation. 150 active TB patients, 150 household contacts and 150 healthy controls were recruited from North Indian population for the study after obtaining written informed consent.
The study was conducted adopting the ethical principles stated in the latest version of Helsinki Declaration as well as the applicable guidelines for good clinical practice (GCP). Ethical approval was obtained from Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi (Ref NO: IEC/NP-299/07.08.2015, RP-03/2015). The demographic profile and clinical characteristics of study participants is shown in Table 1
Sample collection and storage
6 mL of peripheral venous blood was collected from patients and controls. The blood specimen was given a personal identifier number that was used to link and maintain the biological information derived. 4 mL blood was for serum separation and was stored at −70 °C for further analysis. 2 mL blood was collected in EDTA vials for the separation of DNA and RNA for genomic and expression studies.
Genotyping for Fok1 VDR Single Nucleotide Polymorphism (SNP)
Genomic DNA was isolated from blood using Relia prep Blood gDNA Miniprep system (A5081), Promega. The Fok1 VDR SNP was performed by PCR-RFLP. PCR was done using Thermo Scientific, #K0171 master mix to amplify the desired sequence of VDR using specific primers. The sequence of forward and reverse primers were as follows:
Forward-(5′-AGCTGGCCCTGGCACTGACTCTGCTCT-3′)
Reverse- (5′-ATGGAAACACCTTGCTTCTTCTCCCTC-3′)
with cycling parameters as follows: Denaturation at 95 °C for 5 min, 35 cycles at 95 °C for 30 s, 65.8 °C for 30 s and 72 °C for 60 s and one final cycle of extension at 72 °C for 5 min. The PCR product (10 µl) of 280 bp was then digested with 1.0 unit of Fok I restriction enzyme (#FD2144, Thermo Scientific) and incubated at 37 °C for 15 min. Genotypic patterns were determined by resolving the digested products on a 2% agarose gel (Figure S1).
Quantitative PCR (qPCR) for expression study
RNA isolation was done from whole blood using TRIzol method and cDNA was synthesized using Verso cDNA synthesis kit (AB1453A, Thermo). Real time detection of VDR was done using above mentioned specific primers of VDR mRNA using 2X Maxima SYBR Green qPCR master mix (Thermo scientific). The PCR amplification was performed in a 10 μl reaction volume with cycling parameters as follows: Denaturation at 95 °C for 5 min, 35 cycles at 95 °C for 30 s, 64 °C for 45 s and 72 °C for 30 s and one final cycle of extension at 72 °C for 5 min. GAPDH was used as a reference gene. A melt curve analysis was performed after every reaction to confirm the specific amplification of products.
Estimation of serum levels of Vitamin D
Serum levels of Vitamin D were estimated for all samples using automated chemiluminiscence based immunoassay system (VITROS ECi, Johnson and Johnson Ortho Clinical Diagnostics). Competitive immunoassay was used where the light signals read by the system is inversely proportional to the concentration of 25-OH vitamin D present in the sample.
Estimation of serum levels of VDR and cathelicidin
Serum levels of VDR was measured using a Human VDR Sandwich ELISA kit (SEA475Hu, USCN, Life Science Inc., Wuhan, Hubei).
Serum levels of cathelicidin was measured using Human LL-37 Sandwich ELISA kit, HK321 (Hycult Biotech, Netherlands).
Statistical analysis
All statistical analyses were performed on GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). Non-parametric statistical analyses were performed throughout the study. Kruskal Wallis and Mann-Whitney U test was used for comparison of variables between three and two groups respectively. The polymorphic variants were analysed by chi-square test with values predicted by the Hardy Weinberg equilibrium model. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to quantitatively assess the strength of association between the polymorphisms and TB. Values were expressed as median ± standard deviation. A p-value of less than 0.05 was considered significant.
References
Global tuberculosis report 2018. https://www.who.int/tb/publications/global_report/en/.
Haussler, M. R. et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 13, 324–4 (1998).
Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 311, 1770–3 (2006).
Hewison, M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 7, 337–45 (2011).
Sørensenl, O., Arnljots, K., Cowland, J. B., Bainton, D. F. & Borregaard, N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 90, 2796–2803 (1997).
Agerberth, B. et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 96, 3086–93 (2000).
Sonawane, A. et al. Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell Microbiol. 13, 1601–17 (2011).
Bellamy, R. et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 179, 721–4 (1999).
Liu, W. et al. VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: a case-control study. Int J Tuberc Lung Dis. 8, 428–34 (2004).
Mahmoud, A. A. & Ali, A. H. K. Vitamin D receptor gene polymorphism and 25 hydroxy vitamin D levels in Egyptian patients with pulmonary tuberculosis. Egypt J Chest Dis Tuberc. 63, 651–5 (2014).
Selvaraj, P., Chandra, G., Kurian, S. M., Reetha, A. M. & Narayanan, P. R. Association of vitamin D receptor gene variants of BsmI, ApaI and FokI polymorphisms with susceptibility or resistance to pulmonary tuberculosis. Curr Sci. 84, 1564–1563 (2003).
Babb, C. et al. Vitamin D receptor gene polymorphisms and sputum conversion time in pulmonary tuberculosis patients. Tuberc Edinb Scotl. 87, 295–302 (2007).
Kang, T. J. et al. Vitamin D Receptor Gene TaqI, BsmI and FokI Polymorphisms in Korean Patients with Tuberculosis. Immune Netw. 11, 253–7 (2011).
Kerr, G. et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Molecular and Cellular Endocrinology. 177, 145–159 (2001).
Colin, E. M. et al. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin Endocr. 52, 211–6 (2000).
Singh, I. et al. VDR polymorphism, gene expression and vitamin D levels in leprosy patients from North Indian population. PLoS Negl Trop Dis. 12(11) (2018).
Medapati, R. V., Suvvari, S., Godi, S. & Gangisetti, P. NRAMP1 and VDR gene polymorphisms in susceptibility to pulmonary tuberculosis among Andhra Pradesh population in India: a case-control study. BMC Pulm Med. 17(1), 89 (2017).
Lee, Y. H. & Song, G. G. Vitamin D receptor gene FokI, TaqI, BsmI, and ApaI polymorphisms and susceptibility to pulmonary tuberculosis: a meta-analysis. Genet Mol Res 14, 9118–29 (2015).
Selvaraj, P., Prabhu Anand, S., Harishankar, M. & Alagarasu, K. Plasma 1,25 dihydroxy vitamin D3 level and expression of vitamin D receptor and cathelicidin in pulmonary tuberculosis. J Clin Immunol. 29, 470–8 (2009).
Mandal, D. et al. Vitamin D receptor expression levels determine the severity and complexity of disease progression among leprosy reaction patients. New Microbes New Infect. 6, 35–9 (2015).
Xu, Y. et al. Using a cDNA microarray to study cellular gene expression altered by Mycobacterium tuberculosis. Chin Med J (Engl). 116, 1070–3 (2003).
Sita-Lumsden, A., Lapthorn, G., Swaminathan, R. & Milburn, H. J. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax 62(11), 1003–7 (2007).
Sasidharan, P. K., Rajeev, E. & Vijayakumari, V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 50, 554–558 (2002).
Yamshchikov, A. V. et al. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr. 92, 603–11 (2010).
Kumar, N. P. et al. Heightened circulating levels of antimicrobial peptides in tuberculosis-Diabetes co-morbidity and reversal upon treatment. PLoS One;12(9) (2017).
Adams, J. S. et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182(7), 4289–4295 (2009).
Gonzalez-Curiel, I. et al. Differential expression of antimicrobial peptides in active and latent tuberculosis and its relationship with diabetes mellitus. Hum Immunol. 72, 656–662 (2011).
Acknowledgements
We thank the DOT center, AIIMS and Nehru Nagar and all the study subjects and Department of Biochemistry and Department of Medicine, AIIMS, New Delhi for assistance. We acknowledged Science and Engineering Research Board (SERB) for funding this work. This work was supported by “Young Scientist Scheme, Science and Engineering Research Board, Department of Science and Technology (SERB-DST), India”. Grant number: YSS/2014/000962.
Author information
Authors and Affiliations
Contributions
Archana Singh, Sudhasini Panda and Kalpana Luthra conceptualized and designed the study. Sudhasini Panda drafted the manuscript. Sudhasini Panda and Ambrish Tiwari carried out recruitment of patients under guidance of S.K. Sharma. Sudhasini Panda and Ambrish Tiwari carried out sample collection, standardisation and execution of experimental work along with data acquisition and interpretation of data under guidance of Archana Singh. Kalpana Luthra, Archana Singh and S.K. Sharma critically reviewed and contributed to the final version of manuscript. Archana Singh gave the final approval of manuscript submission and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panda, S., Tiwari, A., Luthra, K. et al. Association of Fok1 VDR polymorphism with Vitamin D and its associated molecules in pulmonary tuberculosis patients and their household contacts. Sci Rep 9, 15251 (2019). https://doi.org/10.1038/s41598-019-51803-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51803-8
This article is cited by
-
Interaction between Fokl polymorphism and vitamin D deficiency in the symptoms of mental disorders in adults: a population-based study
Scientific Reports (2024)
-
Protective Role of Vitamin D Against Development of Active Tuberculosis in Close Household Contacts of Pulmonary Tuberculosis Patients: A Prospective Cohort Study
Indian Journal of Clinical Biochemistry (2024)
-
Increased susceptibility to complicated pneumonia among egyptian children with FokI (rs2228570), not TaqI (rs731236), vitamin D receptor gene polymorphism in association with vitamin D deficiency: a case-control study
BMC Pediatrics (2023)
-
Tuberculosis: current scenario, drug targets, and future prospects
Medicinal Chemistry Research (2021)
-
Association of genetic variations in the vitamin D pathway with susceptibility to tuberculosis in Kazakhstan
Molecular Biology Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.







