Abstract
Tuberculous empyema (TE) is associated with high mortality and morbidity. In the retrospective cohort study, we aimed to find risk factors for TE among pleural tuberculosis (TB) patients. Between July 2011 and September 2015, all culture-confirmed pleural TB patients (474 cases) were enrolled in our study. Empyema was defined as grossly purulent pleural fluid. Demographic and epidemiological data were collected for further analysis. Multivariate logistic regression analysis was used to evaluate risk factors of TE in pleural TB, age–adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated to show the risk. The mean age was 35.7 ± 18.1 years old, males comprised 79.1% of the participants (375 cases). Forty-seven patients (9.9%) were multidrug-resistant TB (MDR-TB), 29 (6.1%) had retreatment TB, 26 (5.5%) had diabetes mellitus. The percentage of empyema patients was 8.9% (42 cases). Multivariate analysis revealed that male (adjusted OR = 4.431, 95% CI: 1.411, 13.919), pleural adenosine deaminase (ADA, >88 U/L) (adjusted OR = 3.367, 95% CI: 1.533, 7.395) and white blood cell (WBC, >9.52 109/L) (adjusted OR = 5.763, 95% CI: 2.473, 13.431) were significant risk factors for empyema in pleural TB, while pulmonary TB (adjusted OR = 0.155, 95% CI: 0.072, 0.336) was the protective factor for the patients. TE remains a serious threat to public health in China. Male sex is a significant risk factor for TE while the presence of pulmonary TB is protective, and high levels of pleural ADA and WBC count could aid in early diagnosis of TE. This finding would help towards reducing the mortality and morbidity associated with TE.
Similar content being viewed by others
Introduction
Globally, tuberculosis (TB) remains one of the serious public health problems, especially in the high TB burden countries like China. According to WHO, in 2017, an estimated 10.0 million were newly diagnosed with TB worldwide, about 14% of them were extrapulmonary TB (EPTB), and 160 000 cases were multidrug-resistant TB (MDR-TB), which is defined as resistance to at least rifampicin and isoniazid1. Recently, a survey study in Tianjin, China demonstrated that pleural TB was the most common type of EPTB and accounted for two-thirds of all EPTB cases2. Therefore, it is thought that pleural TB may be frequent in high-incidence TB countries3. Despite treatment, pleural TB may progress to tuberculous empyema (TE), which can cause a chronic and fatal sequelae4.
TE is an uncommon form of pleural TB. It is characterized as a purulent infection occupying the pleural space and bacilli can be found in pleural effusion (PE)5. According to the American Thoracic Society, an empyema refers to a collection of pus in the pleural space and could be classified into three progressive stages, namely the exudative (I), fibrinopurulent (II) and organizing (III) phrases6. Over the past few decades, the incidence of TE has decreased significantly, but it still threatens public health7. Although this situation is changing with the development of medical treatment (such as potent anti-TB medication, punctures/drainage, and appropriate intrapleural antifibrinolytics), however, the increasing HIV epidemic would increase the risk of TB in developing countries8,9, and could reverse the trend. Moreover, for empyema, the mortality rate among HIV-infected patients is higher than that of non-infected patients (26.2% vs 5.1%)10.
Untreated or inadequately treated TE may result in a pleurocutaneous fistula (empyema necessitatis), chest wall mass, and rib and bone destruction11. Moreover, the development of TE is associated with high mortality and morbidity6. It is suggested that, in the UK, 20% of patients with empyema die, and approximately 20% require surgery to achieve cure within 1-year of initial infection12,13. Several factors, such as chronic respiratory diseases, diabetes mellitus, malignancies, immunosuppression, gastro-oesophageal reflux disease and alcohol and drug addiction, are found to increase the risk of progression to empyema14. However, risk factors for TE remains unclear. In the retrospective cohort study, we aimed to evaluate risk factors for TE among pleural TB patients.
Materials and Methods
The retrospective study was performed at the Shandong Provincial Chest Hospital (SPCH), located in Jinan city in eastern China. SPCH is a provincial referral TB hospital of approximately 800 beds. Each year, about 500 patients with pleural TB are diagnosed, most of them are diagnosed clinically, not confirmed by culture or pathological examination. In Shandong Province, which has a population of 90 million, 40, 000 new TB cases are found annually and about 10% isolates are MDR-TB15. This study was approved by the SPCH Ethics Committees. Under Article 39.1 of ethics guidelines enacted by National Health and Family Planning Commission of the PRC (http://www.gov.cn/gongbao/content/2017/content_5227817.htm), this study was exempt from the need for written informed consent, as it used only secondary data.
Between July 2011 and September 2015, consecutive culture-confirmed pleural TB patients were enrolled in our study. Empyema was defined as grossly purulent pleural fluid16. Demographic and epidemiological data were collected using a questionnaire from electronic medical records, including sex, age, contact history of TB, smoking habit, treatment delays, underlying diseases and symptoms, and then were analyzed as described previously17.
Mycobacterium tuberculosis (M.TB) strains underwent drug susceptibility testing by using the absolute concentration method (isoniazid: 1 μg/ml, rifampicin: 50 μg/ml) on Lowenstein-Jensen medium15. PE samples obtained by thoracocentesis underwent biochemical and microbiological analysis. An automated chemistry analyzer (Advia 2400, Siemens Healthcare Diagnostics, Tokyo, Japan) was used to examine biochemical parameters, such as total protein, total bilirubin, glucose, lactate dehydrogenase (LDH) and adenosine deaminase (ADA). Hematological tests and flow cytometry analysis were performed on the EDTA whole blood, using a XT-1800i hematology analyzer (Sysmex Corporation, Kobe, Japan) and FACSCalibur Flow Cytometer (BD Biosciences, San Diego, USA), respectively.
Patient characteristics were summarized using means and standard deviations (or median and interquartile range) for continuous variables and counts/percentages for categorical variables. Categorical variables were compared using the χ2 test or Fisher exact test, and continuous variables compared using the Mann-Whitney U test or t test depending on the distribution of the data. Beyond descriptive statistics, associations between TE and clinical characteristics were analyzed by calculating the odds ratios (OR) and 95% confidence interval (CI), adjusted by age. Univariate logistic regression analysis was used to evaluate risk factors for TE, and significant variables (P < 0.1) were included in the multivariate logistic regression model. Prior to conducting multivariate logistic regression, continuous variables were transformed into categorical variables based on the cutoff points determined with receiver operating characteristic curve (ROC) analysis. Multivariate logistic regression analysis was then performed, including significant variables estimated in the univariate analysis or variables suggested in the literature. The Hosmer-Lemeshow goodness-of-fit test was performed to assess the overall fit of the model. A two-sided P value < 0.05 was considered significant for all analyses. Data analysis was carried out using SPSS 16.0 (IBM Corp., Armonk, United States).
Results
Characteristics of patients
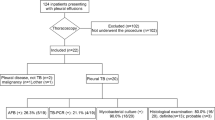
Table 1 shows the demographic characteristics of the 474 participants of this study. All 474 pleural TB patients (Additional File 1) enrolled were culture-confirmed, and 42 of them (8.9%) were diagnosed as TE because of an accumulation of pus in the pleural space. The mean age was 35.7 ± 18.1 years old, males comprised 79.1% of the participants. Forty-seven patients (9.9%) were MDR-TB. Forty-nine (10.3%) patients had contact of TB history. Smokers constituted 51.3% of the participants. The delay period of treatment was 94.3 ± 237.6 days. Among them, 344 (72.6%) participants had pulmonary TB, 70 (14.8%) had EPTB (excluding pleural TB), 29 (6.1%) had retreatment TB, 26 (5.5%) had diabetes mellitus, 4 (0.8%) had milliary TB. Cough (71.1%) was the most commonly reported symptom. Fever (70.7%), dyspnea (51.5%), chest pain (41.4%), sputum production (37.6%), and fatigue (8.2%) were the other symptoms. Chest X-ray revealed that 56.8% of the effusions were on the right, 33.8% on the left and 9.5% on the both.
The results of PE biochemical tests, flow cytometry analysis and hematological tests were also summarized in Table 2 and Supplementary Table 1. For comparisons between groups in terms of continuous variables, Mann-whitney U tests showed that differences in glucose (P < 0.001), LDH (P < 0.05), ADA (P < 0.05), red blood cell count (P < 0.01), hemoglobin (P < 0.01), hematocrit (P < 0.01) and erythrocyte sedimentation rate (P < 0.05) were significant when comparing the two groups. The t tests showed that age (P < 0.05) and white blood cell count (WBC, P < 0.05) were significantly different between the two groups. The other continuous variables did not reach significance (all P > 0.05). For dichotomous variables, chi-square analysis of these data showed that the two groups were significantly different in terms of pulmonary TB (P < 0.001), dyspnea (P < 0.05) and effusion site (right, P < 0.05). The other categorical variables did not reach significance (all P > 0.05).
Univariate and multivariate analysis
Tables 1 and 2 shows the univariate analysis of risk factors, comparing patients with empyema with patients without empyema. The presence of empyema was associated with age (OR = 0.973, 95% CI: 0.951, 0.995), pulmonary TB (OR = 0.244, 95% CI: 0.127, 0.467), dyspnea (OR = 2.000, 95% CI: 1.025, 3.903), effusion site (right, OR = 2.297, 95% CI: 1.125, 4.689), pleural biochemical tests (glucose (OR = 0.802, 95% CI: 0.697, 0.922), LDH (OR = 1.000, 95% CI: 1.000, 1.000) and ADA (OR = 1.009, 95% CI: 1.004, 1.013)) and hematological tests (WBC (OR = 1.123, 95% CI: 1.025, 1.232), red blood cell (OR = 2.385, 95% CI: 1.332, 4.270), hemoglobin (OR = 1.023, 95% CI: 1.005, 1.041), hematocrit (OR = 1.098, 95% CI: 1.026, 1.176) and erythrocyte sedimentation rate (OR = 0.985, 95% CI: 0.972, 0.998)) (all P < 0.05).
To make the results as readily understandable as possible, continuous variables were converted into dichotomous categorical variables based on the cutoff points determined with ROC analysis, and the corresponding optimal cutoff values were 88 U/L and 9.52 109/L for ADA or WBC, respectively. Further multivariate analysis (Hosmer–Lemeshow goodness-of-fit test: χ2 = 2.329, df = 5, P = 0.802) revealed that male (adjusted OR = 4.431, 95% CI: 1.411, 13.919), pleural ADA (>88 U/L) (adjusted OR = 3.367, 95% CI: 1.533, 7.395) and WBC (>9.52 109/L) (adjusted OR = 5.763, 95% CI: 2.473, 13.431) were significant risk factors for empyema in pleural TB, while pulmonary TB (adjusted OR = 0.155, 95% CI: 0.072, 0.336) was the protective factor for the patients (Table 3, Additional File 2).
Discussion
Currently, the pathogenesis of empyema is not well understood18. TE, is less common and represents an infection of the pleural space by M.TB that provokes the accumulation of purulent pleural fluid19. TE usually occurs in younger and middle-aged patients20. However, the accurate incidence has not been properly evaluated recently. In a previous study, TE has been reported that accounts for 3%–6% of all cases of empyema in South Korea21. In this study, we conducted a retrospective cohort study to evaluate the risk factors of TE in pleural TB patients. To our best knowledge, this is the first report detailing risk factors for the incidence of TE among pleural TB patients in China.
Our results indicated that pulmonary TB is a protective factor for TE. TE usually develops due to chronic pulmonary TB22, and rarely as a result of untreated tuberculous pleural effusion (TPE)23. Prior to the diagnosis of TE, patients often have suffered from chronic pulmonary TB for more than 10 years6. This is due to the fact that most of TE patients are asymptomatic24. Nevertheless, sputum culture positive for M.TB is also considered as a gold standard criteria for TPE diagnosis25. Therefore, the TE secondary to pulmonary TB would be more easily detected, because of the additional specimen (sputum) examined. Then, anti-TB drugs would be given timely, in other words, this may have a positive effect on the prognosis of TE patients. In addition, Ornstein GG et al. suggested that, when the underlying pulmonary TB is under control, the prognosis of TE is good; nevertheless, when the associated pulmonary TB not under control, the prognosis is not good26. This also indicates that an increased association between pulmonary TB and TE outcomes is plausible.
In the study, we found that male sex was a risk factor for TE. A possible explanation for it is that sex imbalance exists in TPEs27,28. Das DK et al. analyzed the sex distribution of TPE patients included in the previous published literatures, and most of studies (3/20) showed that female patients constituted the majority of TPEs (>50%)28. In the United States, from 1993 through 2003, 7,549 cases of pleural TB were reported, males comprised 67% and females 33% of the patient population. A similar result was reported in a retrospective study of 254 pleural TB patients29.
High levels of pleural ADA and peripheral WBC count predict increased TE risk. The findings suggest that the two biomarkers may reveal the progression of TE. Several reports have discovered the diagnostic role of the two in patients with empyma. Li R et al. reported that PE ADA could be used as an alternative biomarker for early and quick discrimination of Gram-negative from Gram-positive bacterial infections of the pleural space, which is useful for the selection of antibiotics30. A retrospective study conducted by Porcel JM et al. concluded that a high level of ADA is a general characteristic of lymphocytic and neutrophilic TB effusions, and an extremely high ADA activity is usually considered in empyema or lymphoma31. For the treatment of empyema, the stage of its development is the main parameter to be considered. A variety of treatments, including appropriate antibiotic therapy, pleural drainage, decortications, thoracotomy and video-assisted thoracoscopic surgery, must be selected and combined together in the most appropriate way with optimal timing32. However, currently there is no biomarker available that would help to choose appropriate interventions33. Thus, careful consideration should be given to the indications of each therapy for critical patients. In the next study, we would examine whether the pleural ADA level and peripheral WBC count could help to identify stage II–III empyema thoracis.
The study was conducted in a large population, and the findings represent a valuable contribution to the field. However, several limitations in the study are worth noting. First, retrospective nature could not control patients’ baseline characteristics, so the results might be subject to selection bias. Second, as you know, the stage of empyema is considered as a main factor for selection of appropriate treatment approach. If an association between empyema stages and these clinical-pathological characteristics is observed, it may aid to control the development of TE. However, we failed to investigate it, this was because of the lack of information on the empyema stage of each patient. Third, because of a single-center design, the findings may be limited which may not accurately reflect the general characteristics of TE in China. In addition, although several risk factors were identified, further analysis must be performed to validate our findings.
Conclusions
Our study found that male sex is a significant risk factor for TE while the presence of pulmonary TB is protective, with high levels of pleural ADA and WBC count could aid in early diagnosis of TE. Given the high proportion of TE in pleural TB was reported, TE remains a serious threat to public health in China.
References
WHO: Global tuberculosis report 2018. 2018. https://www.who.int/tb/publications/global_report/en/. Accessed 7 March (2019).
Wang, X., Yang, Z., Fu, Y., Zhang, G. & Zhang, Y. Insight to the Epidemiology and Risk Factors of Extrapulmonary Tuberculosis in Tianjin, China during 2006–2011. PLoS One 9, e112213 (2014).
Vorster, M. J., Allwood, B. W., Diacon, A. H. & Koegelenberg, C. F. Tuberculous pleural effusions: advances and controversies. J Thorac Dis 7, 981–991 (2015).
Yang, L. et al. Analysis of Cytokine Levers in Pleural Effusions of Tuberculous Pleurisy and Tuberculous Empyema. Mediators Inflamm 2016, 3068103 (2016).
Bryant, R. E. & Salmon, C. J. Pleural empyema. Clin Infect Dis 22, 747–762; quiz 763–744 (1996).
Al-Kattan, K. M. Management of tuberculous empyema. Eur J Cardiothorac Surg 17, 251–254 (2000).
Akgul, A. G., Orki, A., Orki, T., Yuksel, M. & Arman, B. Approach to empyema necessitatis. World J Surg 35, 981–984 (2011).
Rabiou, S. et al. A Simplified Technique for Drainage of Chronic Calcified Pleural Empyema. Thorac Cardiovasc Surg 65, 586–588 (2017).
Porco, T. C., Small, P. M. & Blower, S. M. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr 28, 437–444 (2001).
Dagnra, A. Y. et al. Incidence and impact of HIV infection among patients with bacterial pleurisy in Lome (Togo). Med Mal Infect 34, 216–220 (2004).
Rossi, S. E., Erasmus, J. J. & McAdams, P. H. Thoracic Manifestations of Tuberculosis. Contemporary Diagnostic Radiology 23, 1–8 (2000).
Ferguson, A. D., Prescott, R. J., Selkon, J. B., Watson, D. & Swinburn, C. R. The clinical course and management of thoracic empyema. QJM 89, 285–289 (1996).
Davies, H. E., Davies, R. J. & Davies, C. W. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65(Suppl 2), ii41–53 (2010).
Teh, E. & Belcher, E. Core Topics in Thoracic Surgery: Surgical aspects of infectious conditions of the lung. (2016).
Deng, Y. et al. Laboratory-based surveillance of extensively drug-resistant tuberculosis, China. Emerg Infect Dis 17, 495–497 (2011).
Berger, H. A. & Morganroth, M. L. Immediate drainage is not required for all patients with complicated parapneumonic effusions. Chest 97, 731–735 (1990).
Zhang, W., Han, C., Wang, M. S. & He, Y. Characteristics and factors associated with treatment delay in pleural tuberculosis. QJM (2018).
Redden, M. D., Chin, T. Y. & van Driel, M. L. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 3, CD010651 (2017).
Ferrer, J. Tuberculous pleural effusion and tuberculous empyema. Semin Respir Crit Care Med 22, 637–646 (2001).
Fang, Y., Xiao, H., Sha, W., Hu, H. & You, X. Comparison of closed-chest drainage with rib resection closed drainage for treatment of chronic tuberculous empyema. J Thorac Dis 10, 347–354 (2018).
Jess, P., Brynitz, S. & Friis Moller, A. Mortality in thoracic empyema. Scand J Thorac Cardiovasc Surg 18, 85–87 (1984).
Sekiya, K., Sakai, T., Homma, S. & Tojima, H. Pulmonary tuberculosis accompanied by a transient increase in serum carcinoembryonic antigen level with tuberculous empyema drainage. Intern Med 46, 1795–1798 (2007).
Long, R., Barrie, J. & Peloquin, C. A. Therapeutic drug monitoring and the conservative management of chronic tuberculous empyema: case report and review of the literature. BMC Infect Dis 15, 327 (2015).
Jenssen, A. O. Chronic calcified pleural empyema. Scand J Respir Dis 50, 19–27 (1969).
Garcia-Zamalloa, A. & Taboada-Gomez, J. Diagnostic accuracy of adenosine deaminase and lymphocyte proportion in pleural fluid for tuberculous pleurisy in different prevalence scenarios. PLoS One 7, e38729 (2012).
Ornstein, G. G. & Herman, M. The management of pure tuberculous and mixed infection empyemata. Dis Chest 12, 1–25 (1946).
Klimiuk, J., Safianowska, A., Chazan, R., Korczynski, P. & Krenke, R. Development and Evaluation of the New Predictive Models in Tuberculous Pleuritis. Adv Exp Med Biol 873, 53–63 (2015).
Das, D. K. Age and sex distribution in malignant and tuberculous serous effusions: A study of 127 patients and review of the literature. Geriatr Gerontol Int 15, 1143–1150 (2015).
Valdes, L. et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 158, 2017–2021 (1998).
Li, R., Wang, J., Wang, X. & Wang, M. Pleural effusion adenosine deaminase: a candidate biomarker to discriminate between Gram-negative and Gram-positive bacterial infections of the pleural space. Clinics (Sao Paulo) 71, 271–275 (2016).
Porcel, J. M., Esquerda, A. & Bielsa, S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med 21, 419–423 (2010).
Kern, L., Robert, J. & Brutsche, M. Management of parapneumonic effusion and empyema: medical thoracoscopy and surgical approach. Respiration 82, 193–196 (2011).
Shen, K. R. et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 153, e129–e146 (2017).
Acknowledgements
We thank Dr. Zhang Wei (Linyi People’s Hospital) for her help with the manuscript, especially the methods section.
Author information
Authors and Affiliations
Contributions
W.M.S. and H.Y. conceived and designed the study, interpreted the analysis and contributed to writing the manuscript. W.P. and W.M. conducted the study and collected the data, H.C. contributed to data management analysis and interpretation. The questionnaire was developed by W.M. and W.M.S., and implemented by W.M. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wen, P., Wei, M., Han, C. et al. Risk factors for tuberculous empyema in pleural tuberculosis patients. Sci Rep 9, 19569 (2019). https://doi.org/10.1038/s41598-019-56140-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56140-4
This article is cited by
-
The efficacy and safety of uniportal video-assisted thoracic surgery on the treatment for stage II-III tuberculous empyema: a single-arm clinical retrospective study from 2016 to 2021 in a thoracic surgery center in China
BMC Pulmonary Medicine (2022)
-
Diversity of adenosine deaminase in children with EBV-related diseases
Italian Journal of Pediatrics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.