Abstract
One of the key determinants of a haematophagous vector’s capacity to transmit pathogens is its selection of which host to secure a blood meal from. This choice is influenced by both intrinsic (genetic) and extrinsic (environmental) factors, but little is known of their relative contributions. Blood fed Anopheles mosquitoes were collected from a malaria endemic village in Ghana. Collections were conducted across a range of different host availabilities and from both indoor and outdoor locations. These environmental factors were shown to impact dramatically the host choice of caught malaria vectors: mosquitoes caught indoors were ten-fold more likely to have sourced their blood meal from humans; and a halving in odds of being human-fed was found for mosquitoes caught only 25 m from the centre of the village. For the first time, we demonstrate that anthropophagy was better explained by extrinsic factors (namely, local host availability and indoor/outdoor trapping location) than intrinsic factors (namely, the (sibling) species of the mosquito caught) (respective Akaike information criterion estimates: 243.0 versus 359.8). Instead of characterizing biting behaviour on a taxonomic level, we illustrate the importance of assessing local entomology. Accounting for this behavioural plasticity is important, both in terms of measuring effectiveness of control programmes and in informing optimal disease control strategies.
Similar content being viewed by others
Introduction
Mosquitoes are responsible for the transmission of multiple human pathogens with malaria the most prominent of the resulting diseases. Malaria is transmitted by the bite of several Anopheles mosquito species when infected with Plasmodium parasites. As the successful transmission of the parasite requires two successful bites (one for the mosquito to become infected and one for the parasite to be transmitted to the human host), the mosquito’s choice of which host species to bite has an exaggerated, non-linear impact on malaria transmission intensity1. The way in which host availability impacts the choice of host is largely unknown despite the dramatic impact it has on transmission and control of vector-borne diseases of diverse aetiology2. Similarly little is known of the short- and long-term impacts of different control methods on host choice.
The introduction of indoor residual spraying and insecticidal nets has resulted in a change in the biting behaviour of major malaria vectors with many mosquito species seeking a higher proportion of blood meals outdoors3,4,5,6 and/or from non-human hosts7,8,9,10. Coupled with increasing rates of insecticide resistance11, these behavioural changes indicate potential inadequacies of current leading vector-control tools to target outdoor biting and, correspondingly, sustained residual malaria transmission12,13,14,15,16,17,18,19. Therefore, understanding this behaviour is becoming increasingly important when implementing vector control strategies.
In the field, which host is ultimately bitten is a complex balance between intrinsic (genetic) and extrinsic (environmental) factors including behavioural conditioning driven by exposure to insecticides or previous successful feeds from a particular host20,21. The amalgamation and balance of these factors results in a significant level of host biting plasticty which can have considerable consequences for disease control2. One of the most popular methods of investigating host biting behaviour in the field is to collect blood fed mosquitoes and identify the blood meal source. The proportion (or percentage) of blood meals that are of human origin is referred to as the Human Blood Index (HBI)22. Malaria-transmitting mosquitoes are often described as primary or secondary vectors with this categorisation informed, in part, by their level of anthropophagy as indicated by their HBI. Owing to the influences of different environmental settings, however, studies reporting HBI for the same vector species demonstrate considerable variability21,23,24,25. We recently showed that the HBI of Anopheles coluzzii varied significantly over an extraordinarily small spatial scale, thus demonstrating how localised host biting behaviour can be26. Further, a recent systematic review indicated that the location a mosquito was caught (indoors versus outdoors) may have just as much influence, if not more influence, on host selection than the taxa collected23. However, these studies were performed by multiple researchers using a variety of methods on different mosquito populations, and spanned several decades. To the best of our knowledge, the relative influence of intrinsic and extrinsic factors on host selection has not been reported for any major disease vector.
In this study, multiple species of blood fed Anopheles mosquitoes were collected across a range of host availabilities, both from indoors and outdoors. Our hypothesis was that local environmental factors had a greater influence over blood host selection than the (sibling) species of the caught mosquitoes. We discuss the consequences of our findings to assessing, and perhaps even augmenting, future control strategies.
Methods
Study site and mosquito collection
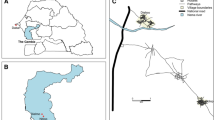
Mosquitoes were collected from the village of Dogo, in the Greater Accra region of Ghana (05°52.418N, 00°33.607E) over 22 nights across June and July 2018 (Fig. 1). The village is on the south-eastern coast of Ghana, with a main rainy season from April to June and a shorter second rainy season in October. Temperatures were measured using a datalogger (EasyLog USB, UK) with windspeed recorded using an anemometer (Holdpeak, UK). Housing mostly consisted of concrete structures with concrete/brick walls and flooring, with predominantly iron roofs although some traditional mud style houses were also present. Several criteria were used to select the site: it had a defined area where cattle were kept and rested during the collection period on the outskirts of the village; the village had no other nearby cattle holdings; human housing density gradually increased towards the village centre and away from the cattle pen. Critical for this site being selected was that it offered both indoor and outdoor mosquito collection points within a full range of availabilities of alternative host species. Mosquito species known to be present in the area were primarily An. gambiae and An. coluzzii as well as An.pharoensis and An. rufipes. Due to the proximity to the sea, the potenital of An. melas being present was also noted and considered during molecular analysis.
Outdoor mosquito collections consisted of three outdoor trapping types: Centers for Disease Control and Prevention (CDC) resting traps (Bioquip products, CA, USA), BG Sentinel 2 traps (Biogents AG, Regensburg, Germany) and CDC light traps (Bioquip products, CA, USA). These traps were placed in clusters (<10 m apart) at 50 m intervals to form a 250 m transect comprising of six trapping points (Fig. 1). The transect began at an area of zero human population density (termed ‘T1’) outside of Dogo village and nearby the cattle resting and overnight holding pen. The transect extended towards a human population ending at an area of high human density, the middle of Dogo village (denoted ‘T6’). Details of hosts located at each transect point are described in Table 1. Mosquitoes were collected overnight from 6 pm to 6 am. Indoor collections were performed using a Prokopack aspirator (John.W.Hock, FL, USA) at two houses per transect point within the village (T3–T6) with each house being alternated for each collection night. Indoor collections at T1 and T2 were performed in outbuildings, which had no residents. Indoor collections were performed for 15 minutes in each building between 4 am and 6 am of each collection night. Bednets were absent from most households and only seen to be used sporadically in remaining households during sampling.
Ethical clearance
The study was reviewed and cleared by the London School of Hygiene & Tropical Medicine (LSHTM) ethics committee (LSHTM ethics reference: 15216). Ethical clearance was also obtained in country and cleared by the University of Ghana (UOG) ethics committee (University of Ghana, Noguchi Memorial Institute reference: DF22). Ethical clearance was obtained prior to any part of the study being performed. All methods in this study were performed in accordance with all relevant guidelines and regulations.
Recruitment of households for indoor collection
Households within 10 metres of each outdoor trap cluster were approached to be recruited into the study. Households were provided with a participant information sheet explaining the study and for those who could not read or speak English, a local translator was present. If the head of the household agreed to take part in the study, informed consent was provided via the signature of a consent form and the household was recruited into the study. All households were allowed to withdraw at any time during the study.
Morphological identification of mosquito species
Anopheles pharoensis and An. rufipes are morphologically distinguishable from the An. gambiae complex as well as from each other. Morphological Identification of these species was performed in the field prior to sample processing using a key developed by Gillies and Coetzee27. An. gambiae were identified only to the species complex until molecular analysis.
Sella staging of blood-fed mosquitoes
The Sella score is a visual measure of blood meal digestion and is comprised of seven stages. Stage two (II) is a freshly blood fed mosquito and stage seven (VII) is a fully gravid mosquito, stages between are defined by set criteria. Each blood fed mosquito in this study was assessed under light microscope and scored using the Sella score originally described by Detinova28.
Sample storage
Anopheles mosquitoes collected from each transect point were immediately killed using chloroform to stop any active blood meal digestion. Mosquitoes where then sorted with all blood-fed females being processed first. All overtly blood-fed Anopheles mosquitoes were processed individually, noting their Sella score28 (a visual measure of blood meal digestion), transect location, trap type, night collected and collection location (indoor or outdoor). All blood-fed mosquitoes were preserved in RNAlater® (Thermo Fisher Scientific, Life Technologies,UK) in a 96 well plate and stored at 4 °C and transported in this format back to the UK. All samples were stored at −70 °C once transported back to laboratories at the London School of Hygiene & Tropical Medicine.
DNA extraction
DNA was extracted from each Anopheles mosquito individually. Each sample was firstly homogenised using a Qiagen TissueLyser II (Qiagen, UK) with a 5 mm stainless steel bead (Qiagen, UK) and placed in each sample tube in a 96 well plate format (Qiagen UK). Once homogenised, DNA was extracted using the Qiagen DNeasy 96 kits (Qiagen, UK) following the manufacturer’s protocol. Extracted DNA was stored at −20 °C until analysis was performed.
An. gambiae species complex identification
Mosquito species identification first involved a real-time multiplex PCR assay targeting the rRNA gene developed by Bass et al.29. Standard forward and reverse primers were used in conjunction with two species-specific Taqman© probes. The reaction conditions were as follows: a 12.5 µl reaction containing 1 µl of genomic DNA. 6.25 µl of QuantiNova (Qiagen, UK) probe master mix and 2.9 µl of DNA free H2O. 800 nM of forward and reverse primers (Thermo Fischer Scientific, UK), 200 nM of An. arabiensis probe (Sigma-Aldrich, UK) and 80 nM of An. gambiae probe (Applied Biosystems, UK). Samples were run on a Stratagene MX3005P (Agilent Technologies, USA) using cycling conditions of 10 min at 95 °C, followed by 40 cycles of 95 °C for 25 s and 66 °C for 60 s. The increases in fluorescence were monitored in real time by acquiring at the end of each cycle. Analysis was carried out using the Stratagene MxPro software.
An. coluzzii and An. gambiae sensu stricto identification
To differentiate between An. coluzzii and An. gambiae s.s. within the An. gambiae species complex, a single end-point PCR was performed. This PCR targets the SINE200 retrotransposon and utilising an insertion in this area allows the two species to be distinguished following gel visualisation30. An. coluzzii produces a band at 479 bp with An. gambiae producing a band at 249 bp. Reaction was as follows: a 25 µl reaction containing 0.5 mM of forward and reverse primers, 12.5 µl of Hot start Taq polymerase (New England Biolabs NEB, UK), 9.5 µl of nuclease-free water and 2 µl of template DNA. Cycling conditions were as follows: 10 min at 94 °C followed by 35 cycles of 94 °C for 30 s, 54 °C for 30 s, 72 °C for 60 s, a final elongation step of 72 °C for 10 minutes finished the cycling program.
An. melas identification
An. melas has been found in coastal areas of Ghana. With the study site close to the sea and <5 km from saltwater lagoons, an endpoint assay developed by Scott et al.31 was used to identify this An. gambiae sibling species. The PCR targets the ribosomal rDNA gene and used a universal forward primer with species-specific reverse primers to produce different product sizes allowing species to be identified. The product size was 464 bp for An. melas. Reaction volume consisted of 10 µl of Hot start Taq polymerase (New England Biolabs NEB, UK), 2 µl of universal forward primer (10 µM) 2 µl of An. melas specific reverse primer (10 µM), 4 µl of DNA/RNA free H2O and 2 µl of template DNA. Cycling conditions were as follows: 10 mins at 95 °C followed by 30 cycles of 30 s at 95 °C, 30 s at 50 °C and 30 s at 72 °C and a final elongation stage of 5 mins at 72 °C.
End-point PCR gel visulisation
All endpoint PCR products were visualised on a 2% agarose gel cartridge using an Egel E-Gel iBase Power System and E-Gel Safe Imager Real-Time Transilluminator (Invitrogen, UK).
Blood meal identification
Due to the nature of the experimental set up with humans and bovines dominating all blood meal sources, samples were initially screened using bovine and human specific primers developed by Gunathilaka et al.32. The reaction conditions consisted of a 10 µl reaction including 0.5 M of forward and reverse primers (Integrated DNA Technologies), 5 µl of SYBR green master mix (Roche, UK), 2 µl of nuclease-free water (Roche, UK) and 2 µl of template DNA. PCR was run on a LightCycler 96 real-time PCR machine (Roche, UK) under the following cycling conditions: pre-incubation of 95 °C for 600 s, 40 cycles of 95 °C for 10 s, 62 °C for 10 s and 72 °C for 30 s followed by a melting analysis to determine specific amplification. Any potential human positive samples were confirmed using the Promega Plexor® HY Human DNA forensic detection kit (Promega, UK). This assay was performed following manufacturer’s protocol using a Stratagene MX3005P (Agilent Technologies, USA) real-time PCR machine and analysed using the Promega®Plexor Analysis software.
Statistical analysis
For each blood-fed mosquito, several variables were recorded: its species, the night and location (transect point and indoors/outdoors) of its capture, Sella stage and source host species of its blood meal. Wind speed and rainfall were also recorded for each capture night. Logistic regression was used to test the association between source species of blood meal (bovine vs non-bovine, and, human vs non-human) and i) species of mosquito, ii) transect point and iii) indoors/outdoors capture. Potential interactions between the variables and wind speed and rainfall were also sought. The Akaike Information Criterion (AIC) was used to estimate the relative quality of statistical models generated using intrinsic versus extrinsic factors for describing the data on blood meal sources. Our previous work identified that the level of blood meal digestion increased significantly the further away from the blood source that mosquitoes were caught26. Therefore, we conducted an ordered logistic regression to ascertain whether the Sella stage (for either bovine-fed or human-fed mosquitoes) varied significantly across the transect. All data analyses were performed in STATA. P values < 0.05 were assumed statistically significant.
Results
Summary of collection
A total of 904 blood fed Anopheles mosquitoes were collected across the 22 trapping nights. Of these, 665 (73.6%) were identified as members of the Anopheles gambiae species complex with 519 identified as An. coluzzii (57.4%), 70 were An. gambiae ss (7.7%) and 32 were An. melas (3.5%). 44 (4.9%) were An. gambiae sensu lato but could not be identified past the species complex level (Table 1). In addition, 108 (11.9%) were An. pharoensis, 54 (6%) were An. rufipes and 77 (8.5%) samples could not be morphologically or genetically identified (Table 2).
A similar number of blood-fed mosquitoes were obtained from indoor (n = 493, 55%) and outdoor (n = 411, 45%) collections. Of the 493 collected indoors, 413 were shown to have blood meal sources of bovine origin (84%), 20 human (4%), nine (2%) of mixed human/bovine origin and 51 (10%) unknown. For outdoor collections, 364 (89%) had bovine blood meal sources, 6 (1%) had human sources, 10 (2%) human/bovine mix and 31 (8%) unknown.
The HBI and Bovine Blood Index (BBI) for each species and collection location is shown in Table 3. An. coluzzii had an overall HBI of 6%, with indoor HBI of 8.5% significantly higher than outdoor HBI of 0.6% (Pearson χ2 = 12.4068, p < 0.001). This general trend was maintained across the different species: overall, mosquitoes caught indoors had a higher HBI (Pearson χ2 = 8.7848, p = 0.003) and lower BBI (Pearson χ2 = 9.0013, p = 0.003).
Impact of local host availability on mosquito host choice
Categorising all blood-fed mosquitoes as human-fed (n = 45) or non-human-fed (n = 859), logistic regression demonstrated a significant impact of the distance (in metres) from the village centre where a mosquito was caught (OR: 0.9790 (95% CI: 0.9749–0.9830), p < 0.001). This equates to approximately halved odds of being human-fed for mosquitoes caught 25 m from the village centre. Keeping human-fed mosquitoes as the response variable, logistic regression was repeated – first using mosquito (sibling) species as the (intrinsic) explanatory variable, then using transect point and trapping location (extrinsic) explanatory variables. Estimates of the Akaike information criterion identified the superiority of the model including extrinsic factors (model with extrinsic factors AIC: 243.0, versus, model with intrinsic factors AIC: 359.8).
Rainfall (yes/no) was not found to have a significant impact on whether or not a blood-fed mosquito sourced its meal from humans (p = 0.484) nor did it significantly modify the impact of indoors versus outdoors captures (p = 0.115). Windy nights (defined as nights with wind speeds recorded over 3 m/s) were also shown not to have a significant impact (p = 0.432) on whether or not a blood-fed mosquito sourced the blood meal from humans. However, windy nights were a significant modifier for whether human-fed mosquitoes were caught indoors versus outdoors (p = 0.041).
For mosquitoes which were bovine-fed (n = 796) or not bovine-fed (n = 108), a significant impact of the distance (in metres) from the cattle holding pen was found (OR: 0.9894 (95% CI: 0.9866–0.9922), p < 0.001). This equates to approximately halved odds of being bovine-fed for mosquitoes caught 50 m from the cattle pen which is an equivalent order of magnitude in difference found when comparing indoor- versus outdoor-caught mosquitoes (OR: 0.5875 (95% CI: 0.3644–0.9472), p = 0.029). Keeping bovine-fed mosquitoes as the response variable, logistic regression was repeated – first using mosquito (sibling) species as the (intrinsic) explanatory variable, then using transect point and trapping location (extrinsic) explanatory variables. Estimates of the Akaike information criterion identified the superiority of the model including extrinsic factors (model with extrinsic factors AIC: 611.3, versus, model with intrinsic factor AIC: 660.6).
Both rainfall and windy nights were negatively associated with mosquitoes having sourced their meals from cattle (respectively, OR: 0.2357 (95% CI: 0.1194–0.4654), p < 0.001; OR: 0.4830 (95% CI: 0.2902–0.8037), p = 0.005). Neither rainfall nor windy nights were a significant modifier for whether bovine-fed mosquitoes were caught indoors versus outdoors (respectively, p = 0.999 and p = 0.292). Ordered logistic regression did not find any significant association between the Sella stage of bovine blood meal digestion and distance from the cattle pen, nor between the Sella stage of human blood meal digestion and distance from the village centre (respectively, p = 0.081 and p = 0.464).
Discussion
Mosquito vectors of human diseases differ markedly in their preference for human hosts21,25,33. This preference is driven by both intrinsic (genetic) and extrinsic (environmental) factors, meaning that the same species of vector may exhibit different host-biting behaviours when confronted with a different setting. Malaria vector species are often described as primary or secondary vectors and this is largely contingent on how much they typically bite humans. However, just how much flexibility a vector exhibits according to its local environment is poorly understood. A recent systematic review indicated that where a mosquito is caught (indoors or outdoors) may be as influential on host selection as which vector taxa is collected23. In a malaria endemic setting of southern Ghana, we sought to quantify the relative influence of extrinsic and intrinsic factors in determining which host species is bitten by local vectors.
Over several weeks during the rainy season, mosquitoes were collected across a range of alternative host (bovine or human) availabilities, both from aspirating indoors and from traps placed outdoors. A general preference for bovine blood hosts was demonstrated by local malaria vectors including sibling species that are considered anthropophagic (An. gambiae s.s. and An. coluzzii). Although atypical, the very low HBI for these vectors (respectively 7% and 6%) is not without precedent; a recent systematic review presented a very wide range of HBI values (~0–100%) found among An. gambiae caught across Africa23. More remarkable was the significant associations found between feeding indices and the extrinsic factors under investigation.
Across the different species found locally, blood-fed mosquitoes caught indoors had a significantly higher HBI and significantly lower BBI than those caught outdoors. Despite being an intuitive result, the number of studies describing both indoor and outdoor blood indices are quite limited; and most of these have sought to document the widely recognised generalist (‘catholic’) biting behaviour of An. arabiensis34,35. Perhaps, therefore, even the paragons of anthropophagy such as An. gambiae s.s. are just as unfussy in their host choice; but, we acknowledge that the result we have found in our field site would need replication elsewhere to develop the evidence base to confirm this speculation.
Regression analysis showed that whether or not a blood-fed mosquito obtained its meal from humans was better predicted by its proximity to humans and whether it was caught indoors than by the species of the mosquito. Similarly, whether or not a blood-fed mosquito obtained its meal from cattle was better predicted by its proximity to cattle and whether it was caught indoors than by the species of the mosquito. Sella staging of the blood-fed mosquitoes showed no significant difference in blood meal digestion across the transect. Hence, mosquitoes were moving freely over the 250 m transect indicating that there was no unobserved barrier between village- and cattle pen-adjacent mosquitoes. In other words, individuals from the same sibling species across the transect likely comprised the same mosquito population and observed differences in behaviour were indeed driven through extrinsic factors. We believe this to be the first evidence from the field demonstrating the high degree of potency that the relative influence of extrinsic factors has on host selection by a major disease vector. Our findings suggest categorizing biting behaviour solely on taxonomy or on a broadly spatial level (by region or country) risks missing the highly localized impact extrinsic factors such as host availability can have on mosquito host choice; and this could have significant public health consequences.
Malaria control is chiefly centered on mosquito control, and mosquito control is largely dependent on exploiting human-biting mosquito behaviour. The two most prominent malaria-control mainstays over the past 20 years in Africa have been insecticidal bednets, which target human-seeking mosquitoes, and indoor residual spray of insecticides, which targets mosquitoes that have recently fed. Historically, effectiveness of new formulations and combinations was assessed solely through measuring epidemiological endpoints (usually malaria incidence). More recently, clustered randomised trials have also incorporated the measurement of entomological endpoints36,37,38, following recognition that this is critical in linking insecticidal deployments with the mechanism(s) of disease control. However, monitoring of biting behaviour which fails to account for extrinsically driven plasticity risks misattribution of the mechanism(s) of disease control which would lead to misleading effectiveness measures and consequently jeopardise the rigour of any extrapolations or projections.
In addition to elucidating the mechanisms of effectiveness of current control trials and programmes, a better understanding of plasticity in host choice could qualitatively change disease control strategy. One burgeoning approach to malaria control is the treatment of blood-hosts with systemic insecticides. These insecticides are delivered topically, orally or parenterally and operate through the uptake of toxic compounds during a bite. They have been demonstrated in trials to significantly reduce malaria incidence39. These drugs are not restricted to human use and several studies have documented their efficacy in controlling mosquito vectors when applied to local livestock40,41. Models have demonstrated when and where this technology could be most successful either as a standalone tool42,43 or as part of an integrated vector management programme44,45. For these projection efforts to be built upon to inform operational control, reliable data are required for describing: how bites are distributed among different host species; how host choice is impacted by local host availability; and, how this behaviour is impacted in the presence of different control measures (e.g. bednets). In the setting of the present study, bednets were seen and used sporadically but also completely absent from some households during sampling. Therefore the host selection plasticity identified among the local vectors here could be exploited by complementing mass bednet deployments (i.e. a time that is arguably when local vectors will try to exploit alternative blood host species) with systemic insecticidal applications in livestock.
Conducting experiments such as those described in the current study but in different epidemiological/entomological settings – including communities before/after mass distribution of LLINs – is an important focus of future work. There is no reason to believe that host choice is always dominated by extrinsic factors – it is important to know how much this behaviour varies in different settings and what are the key drivers. Hopefully the ease with which the experiment is set up; its relatively modest costs; and, the broadly recognised criticality of human biting rate in malaria epidemiology will encourage others to capitalise on our methods to investigate mosquito biting behaviour in other settings. To that end, findings from this first study could be exploited by others in conducting power analyses to determine appropriate sample sizes for their mosquito collections.
Conclusions
Local host availability is a powerful driver for host selection of even the most discerningly anthropophilic malaria vectors. The importance of not characterizing biting behaviour on a taxonomic level but through explicit study of local mosquito populations is demonstrated. A better understanding of plasticity in host choice is critical for attributing disease reductions to the correct control mechanisms and is key to implementing the most effective malaria control strategy.
Data availability
All data generated or analysed during this study are included in this published article.
References
Macdonald, G. The Epidemiology and Control of Malaria (Oxford University Press, 1957).
Yakob, L. How do biting disease vectors behaviourally respond to host availability? Parasites & vectors 9, 468 (2016).
Fornadel, C. M., Norris, L. C., Glass, G. E. & Norris, D. E. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am. J. tropical Med. Hyg. 83, 848–853 (2010).
Gatton, M. L. et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution 67, 1218–1230, https://doi.org/10.1111/evo.12063 (2013).
Moiroux, N. et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 206, 1622–1629, https://doi.org/10.1093/infdis/jis565 (2012).
Russell, T. L. et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10, 80 (2011).
Tedrow, R. E. et al. Anopheles mosquito surveillance in Madagascar reveals multiple blood feeding behavior and Plasmodium infection. PLOS Neglected Tropical Dis. 13, e0007176, https://doi.org/10.1371/journal.pntd.0007176 (2019).
Sousa, C. A. et al. Dogs as a Favored Host Choice of Anopheles gambiae sensu stricto (Diptera: Culicidae) of São Tomé, West Africa. J. Med. Entomology 38, 122–125, https://doi.org/10.1603/0022-2585-38.1.122 (2001).
Bøgh, C., Pedersen, E. M., Mukoko, D. A. & Ouma, J. H. Permethrin‐impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med. Veterinary Entomology 12, 52–59 (1998).
Ndenga, B. A. et al. Malaria vectors and their blood-meal sources in an area of high bed net ownership in the western Kenya highlands. Malar. J. 15, 76 (2016).
Ranson, H. & Lissenden, N. Insecticide Resistance in African <em>Anopheles</em> Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitology 32, 187–196, https://doi.org/10.1016/j.pt.2015.11.010 (2016).
Sherrard-Smith, E. et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proceedings of the National Academy of Sciences, 201820646, https://doi.org/10.1073/pnas.1820646116 (2019).
Durnez, L. & Coosemans, M. In Anopheles mosquitoes: new insights into malaria vectors/Manguin, Sylvie 671–704 (2013).
Govella, N. J. & Ferguson, H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front. Physiol. 3, 199 (2012).
Killeen, G. F. C. controlling and eliminating residual malaria transmission. Malar. J. 13, 330, https://doi.org/10.1186/1475-2875-13-330 (2014).
Kiware, S. S. et al. Simplified models of vector control impact upon malaria transmission by zoophagic mosquitoes. PLoS one 7, e37661 (2012).
Monroe, A. et al. Human behaviour and residual malaria transmission in Zanzibar: findings from in-depth interviews and direct observation of community events. Malar. J. 18, 220, https://doi.org/10.1186/s12936-019-2855-2 (2019).
Muirhead-Thomson, R. The significance of irritability, behaviouristic avoidance and allied phenomena in malaria eradication. Bull. World Health Organ. 22, 721 (1960).
Pates, H. & Curtis, C. Mosquito behavior and vector control. Annu. Rev. Entomol. 50, 53–70 (2005).
Besansky, N. J., Hill, C. A. & Costantini, C. No accounting for taste: host preference in malaria vectors. Trends Parasitology 20, 249–251, https://doi.org/10.1016/j.pt.2004.03.007 (2004).
Takken, W. & Verhulst, N. O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453, https://doi.org/10.1146/annurev-ento-120811-153618 (2013).
Garrett-Jones, C. The Human Blood Index of Malaria Vectors in Relation to Epidemiological Assessment. Bull. World Health Organ. 30, 241–261 (1964).
Orsborne, J. et al. Using the human blood index to investigate host biting plasticity: a systematic review and meta-regression of the three major African malaria vectors. Malar. J. 17, 479, https://doi.org/10.1186/s12936-018-2632-7 (2018).
Killeen, G. F. et al. Measuring, manipulating and exploiting behaviours of adult mosquitoes to optimise malaria vector control impact. BMJ Glob. Health 2, e000212, https://doi.org/10.1136/bmjgh-2016-000212 (2017).
Wolff, G. H. & Riffell, J. A. Olfaction, experience and neural mechanisms underlying mosquito host preference. J. Exp. Biol. 221, jeb157131 (2018).
Orsborne, J. et al. Investigating the blood-host plasticity and dispersal of Anopheles coluzzii using a novel field-based methodology. Parasites & Vectors 12, 143, https://doi.org/10.1186/s13071-019-3401-3 (2019).
Gillies, M. & Coetzee, M. A supplement to the Anophelinae of Africa South of the Sahara. Publ. S Afr. Inst. Med. Res. 55, 1–143 (1987).
Detinova, T. S., Bertram, D. & Organization, W. H. Age-grouping methods in Diptera of medical importance, with special reference to some vectors of malaria (1962).
Bass, C., Williamson, M. S. & Field, L. M. Development of a multiplex real-time PCR assay for identification of members of the Anopheles gambiae species complex. Acta Tropica 107, 50–53 (2008).
Santolamazza, F. et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 7, 163, https://doi.org/10.1186/1475-2875-7-163 (2008).
Scott, J. A., Brogdon, W. G. & Collins, F. H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. tropical Med. Hyg. 49, 520–529 (1993).
Gunathilaka, N., Denipitiya, T., Hapugoda, M., Abeyewickreme, W. & Wickremasinghe, R. Determination of the foraging behaviour and blood meal source of malaria vector mosquitoes in Trincomalee District of Sri Lanka using a multiplex real time polymerase chain reaction assay. Malar. J. 15, 242, https://doi.org/10.1186/s12936-016-1279-5 (2016).
Garrett-Jones, C., Boreham, P. & Pant, C. Feeding habits of anophelines (Diptera: Culicidae) in 1971–78, with reference to the human blood index: a review. Bull. Entomological Res. 70, 165–185 (1980).
Githeko, A., Service, M., Mbogo, C., Atieli, F. & Juma, F. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta. Tropica. 58, 307–316 (1994).
Mahande, A., Mosha, F., Mahande, J. & Kweka, E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 6, 100 (2007).
Gari, T. et al. Malaria incidence and entomological findings in an area targeted for a cluster-randomized controlled trial to prevent malaria in Ethiopia: results from a pilot study. Malar. J. 15, 145, https://doi.org/10.1186/s12936-016-1199-4 (2016).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet 391, 1577–1588, https://doi.org/10.1016/S0140-6736(18)30427-6 (2018).
West, P. A. et al. Indoor Residual Spraying in Combination with Insecticide-Treated Nets Compared to Insecticide-Treated Nets Alone for Protection against Malaria: A Cluster Randomised Trial in Tanzania. PLOS Med. 11, e1001630, https://doi.org/10.1371/journal.pmed.1001630 (2014).
Foy, B. D. et al. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet 393, 1517–1526, https://doi.org/10.1016/S0140-6736(18)32321-3 (2019).
Poché, R. M., Burruss, D., Polyakova, L., Poché, D. M. & Garlapati, R. B. Treatment of livestock with systemic insecticides for control of Anopheles arabiensis in western Kenya. Malar. J. 14, 351, https://doi.org/10.1186/s12936-015-0883-0 (2015).
Ng’habi, K. et al. Mesocosm experiments reveal the impact of mosquito control measures on malaria vector life history and population dynamics. Sci. Rep. 8, 13949, https://doi.org/10.1038/s41598-018-31805-8 (2018).
Yakob, L. Endectocide-treated cattle for malaria control: a coupled entomological-epidemiological model. Parasite Epidemiol. Control. 1, 2–9 (2016).
Imbahale, S. S. et al. Mapping the potential use of endectocide-treated cattle to reduce malaria transmission. Sci. Rep. 9, 5826, https://doi.org/10.1038/s41598-019-42356-x (2019).
Pasay, C. J. et al. Treatment of pigs with endectocides as a complementary tool for combating malaria transmission by Anopheles farauti (s.s.) in Papua New Guinea. Parasites & Vectors 12, 124, https://doi.org/10.1186/s13071-019-3392-0 (2019).
Yakob, L., Cameron, M. & Lines, J. Combining indoor and outdoor methods for controlling malaria vectors: an ecological model of endectocide-treated livestock and insecticidal bed nets. Malar. J. 16, 114 (2017).
Acknowledgements
We would like to thank the community of Dogo for allowing this study to be performed and especially those individuals who allowed us to place traps around their homes. We would also like to thank Issac Adjawutor and Maccarthy Collins for allowing us access to the community and making this work possible. J.O. has an MRC London Intercollegiate Doctoral Training Partnership Studentship. T.W. and C.L.J. are funded through a Wellcome Trust/Royal Society Sir Henry Dale Fellowship (101285/Z/13/Z) awarded to T.W. L.Y. received funds from a Royal Society Research Project (RSG\R1\180203). Y.A.A. has funding from the National Institute of Health, US (R01AI123074). Funding bodies had no role in the design of the study and collection, analysis and interpretation of data nor in writing the manuscript.
Author information
Authors and Affiliations
Contributions
L.Y. and J.O. conceived the study. J.O., A.R.M. and Y.A.A. performed the fieldwork. J.O., C.L.J., M.K. and T.W. performed laboratory analyses. All authors contributed to experimental designs, results interpretation and manuscript drafting. All authors read and approved the final manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orsborne, J., Mohammed, A.R., Jeffries, C.L. et al. Evidence of extrinsic factors dominating intrinsic blood host preferences of major African malaria vectors. Sci Rep 10, 741 (2020). https://doi.org/10.1038/s41598-020-57732-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57732-1
This article is cited by
-
Higher outdoor mosquito density and Plasmodium infection rates in and around malaria index case households in low transmission settings of Ethiopia: Implications for vector control
Parasites & Vectors (2024)
-
Larval habitat stability and productivity in two sites in Southern Ghana
Malaria Journal (2023)
-
Blood-feeding patterns of Anopheles vectors of human malaria in Malawi: implications for malaria transmission and effectiveness of LLIN interventions
Malaria Journal (2022)
-
The resting behavior of malaria vectors in different ecological zones of Ghana and its implications for vector control
Parasites & Vectors (2022)
-
Widespread zoophagy and detection of Plasmodium spp. in Anopheles mosquitoes in southeastern Madagascar
Malaria Journal (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.