Abstract
Laminotomy and transforaminal lumbar interbody fusion (TLIF) is usually used to treat unstable spinal stenosis. Minimally invasive surgery (MIS) can cause less muscle injury than conventional open surgery (COS). The purpose of this study was to compare the degree of postoperative fatty degeneration in the paraspinal muscles and the spinal decompression between COS and MIS based on MRI. Forty-six patients received laminotomy and TLIF (21 COS, 25 MIS) from February 2016 to January 2017 were included in this study. Lumbar MRI was performed within 3 months before surgery and 1 year after surgery to compare muscle-fat-index (MFI) change of the paraspinal muscles and the dural sac cross-sectional area (DSCAS) change. The average MFI change at L2–S1 erector spinae muscle was significantly greater in the COS group (27.37 ± 21.37% vs. 14.13 ± 19.19%, P = 0.044). A significant MFI change difference between the COS and MIS group was also found in the erector spinae muscle at the caudal adjacent level (54.47 ± 37.95% vs. 23.60 ± 31.59%, P = 0.016). DSCSA improvement was significantly greater in the COS group (128.15 ± 39.83 mm2 vs. 78.15 ± 38.5 mm2, P = 0.0005). COS is associated with more prominent fatty degeneration of the paraspinal muscles. Statically significant post-operative MFI change was only noted in erector spinae muscle at caudal adjacent level and L2–S1 mean global level. COS produces a greater area of decompression on follow up MRI than MIS with no statistical significance on clinical grounds.
Similar content being viewed by others
Introduction
Lumbar spinal fusion with conventional open surgery (COS) is an effective method to relieve the symptoms of spinal stenosis and instability. However, COS is associated with extensive iatrogenic paraspinal muscle injury due to direct muscle dissection, denervation, and ischemia during long hours of muscle retraction. The paraspinal muscles, especially the multifidus and erector spinae muscles, are essential for carrying physiological loads and functional movement, and play an important role in the stability of the lumbar spine1. Muscle atrophy after COS occurs frequently, and can result in back pain and failed back surgery syndrome2. Therefore, minimally invasive surgery (MIS) techniques for lumbar spine fusion have become popular because they avoid long incisions and reduce muscle injury1,3,4. However, few studies have examined the differences of postoperative paraspinal muscle changes and outcomes between COS and MIS.
The purpose of this study was to compare the degree of postoperative fatty infiltration in paraspinal muscles and the degree of spinal decompression between COS and MIS based on magnetic resonance imaging (MRI).
Materials and methods
Patients and data collection
This study was approved by Chang Gung Memorial Hospital’s institution review board and all methods were performed in accordance with the relevant guidelines (approval no. 201702031B0). A written informed consent was obtained from every participant. All procedures in this study were performed in accordance with the relevant guidelines and regulations. This study adheres to STROBE statement guidelines (Supplementary Appendix 1).
This was a prospective, non-randomized, cohort study. Patients who underwent 1- or 2-level transforaminal lumbar interbody fusion (TLIF) by COS or MIS at our hospital from February 2016 to January 2017 were enrolled in the study.
Preoperative clinical symptoms included low back pain, sciatica, and variable neurological symptoms. The pathologies for surgery were segmental instability, spondylolisthesis, and disc degeneration disease with herniation and/or spinal stenosis. Patients with previous spine surgery, spine trauma, infection, ankylosing spondylitis, cancer, and congenital spinal deformities were excluded.
The functional scores (visual analogue scale [VAS] pain score, Oswestry Disability index [ODI], and Japanese Orthopedic Association [JOA] score) are collected pre-operation and 1 year post-operation. The plain radiographs (AP, lateral and flexion–extension views) was done 1 year after the surgery. The complete fusion was determined to be achieved if visible interbody osseous bridge had formed around the cage, no radiolucent line between the cage and endplate, and no motion on the dynamic flexion extension radiographs.
Lumbar MRI studies were obtained within 3 months before surgery and 1 year after surgery.
Surgical technique
All patients received laminotomy, TLIF with cage insertion, and fixation with a transpedicle screw fixation device. Bilateral posterolateral fusion (inter-transverse fusion) was performed in addition to TLIF in the COS group. In the COS group, the paraspinal muscles were dissected away from the posterior elements (spinal process, lamina, and facet joints) via a midline approach, and laminotomy was performed at the index level with preservation of the adjacent supra- and inter-spinous ligament. In the MIS group, a fluoroscope-assisted percutaneous instrumentation technique via a para-midline approach and bilateral laminotomy by unilateral medial facetectomy for decompression were performed.
MRI protocol
To avoid strong susceptibility artifacts around the postoperative metallic instruments, the MRI examinations were performed with a 1.5-T MR scanner (GE, Optima MR 450 W). The MR sequences included axial T1-weighted spin-echo (TR range/TE range 400–550/11–12) and axial T2-weighted spin-echo (TR range/TE range 4,000–4,300/95–120) sequences with a matrix size of 288 × 288, field of view of 200 × 170 mm, slice thickness of 4 mm, and interslice gap of 1 mm in the axial planes. The axial images were obtained at intervertebral disc levels horizontal to the endplate of the vertebral bodies from L2–3 to L5–S1. MR images were evaluated on a picture archiving and communication system work station (Centricity PACS, Radiology RA1000 Workstation, GE Healthcare).
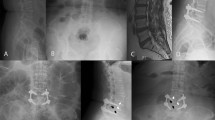
The replacement of lean muscle by a fatty component suggests a decreased contractile muscle component and fatty degeneration of the muscle. The degree of fatty degeneration of the paraspinal muscle was determined by measuring the deposition of fat in the muscle. A muscle-fat-index (MFI) was used to reflect the amount of fatty infiltration in muscle using T1-weighted axial images. The MFI was defined as the mean signal intensity of the target muscle/mean signal intensity of homogenous subcutaneous fat5,6. The regions of interest (ROI) of individual muscles (multifidus muscle and rector spinae muscle) were measured on axial T1-weighted images by placing polygon points around the outer margins of the muscles to avoid metallic artifacts at each disc level from L2–3 to L5–S1, first on postoperative images and then on corresponding preoperative images (Fig. 1). The ROI of homogenous fat tissue was obtained from the homogenous subcutaneous tissue of the back in the same image. The mean signal intensity (SI) in the ROI of the target muscle and fat was obtained for calculating the MFI. The postoperative MFI change = (postoperative MFI − preoperative MFI)/preoperative MFI.
Illustration of the MFI evaluation technique. The mean SI in the ROI of the multifidus muscles (M), erector spinae muscle (E), and subcutaneous fat (F) were obtained from postoperative (a) and preoperative (b) T1WI MRI images using the picture archiving and communication system software tools. MFI mean SI of the target muscle/mean SI of subcutaneous fat.
MFI was measured at the operated level, the cranial adjacent level, the caudal adjacent level, and from L2–3 to L5–S1. The global lumbar spine value was defined as the average of the values obtained from L2–3 to L5–S1.
The dural sac cross-sectional area (DSCSA) was measured at the most stenotic level on preoperative MR images, and at the same level on postoperative MRI T2-weighted axial images by polygon point measurement (Fig. 2). The improvement of spinal stenosis was defined as DSCSA change from before to after surgery.
All measurements were performed independently by 2 different observers who were blinded to the operative method. The average values of the 2 observations at each lumbar level were used for the analysis.
Statistical methods
Comparison of postoperative MFI changes of the multifidus muscle and rector spinae muscle between the COS and MIS groups were performed using the unpaired t test. All statistical comparisons were 2-tailed, and the threshold for statistical significance was set at P < 0.05.
The inter-observer reliability was examined using 1-way analysis of variance, and the intra-class correlation coefficient (ICC).
Results
Initially there were 50 patients enrolled in this study. But 4 patients were excluded due to they did not complete the follow up MRI (2 patient suffered from other medial disease and 2 lost of follow up) (Fig. 3). Finally, total 46 patients (21 in COS group, 25 in MIS group) were included in this study. The mean age of patients in the COS group was 59.2 ± 8.6 years (range 41–77 years), and in the MIS group was 60.2 ± 10.0 years (range 38–78 years). Postoperative lumbar MRI studies were obtained a mean of 12.5 months after surgery in the COS group and 14.7 months after surgery in the MIS group. Patient demographic data, functional scores (visual analogue scale [VAS] pain score, Oswestry Disability index [ODI], and Japanese Orthopedic Association [JOA] score) and interbody fusion rate are summarized in Table 1. There was no significant difference in sex, age, functional scores, and interbody fusion rate between two groups.
The assessment of inter-observer reliability showed good agreement for the MFI (ICC = 0.78), and excellent agreement for the DSCSA measurement (ICC = 0.91), indicating the measurements were reliable.
The postoperative MFI was increased in both the multifidus and erector spinae muscles at all levels in both the COS and MIS groups on the 1-year postoperative MRI studies. The global MFI change of the multifidus muscle at L2–S1 was greater in the COS than the MIS group, but the difference was not statistically significant (12.85 ± 17.17% vs. 7.45 ± 18.76%, P = 0.343). The global MFI change of the erector spinae muscle at L2–S1 was significantly greater in the COS group (27.38 ± 21.37% vs. 14.14 ± 19.19%, P = 0.044) (Fig. 4).
The MFI changes at the operated and the cranial and caudal adjacent levels were compared between the two groups (Fig. 5). There was a greater trend of increasing fat infiltration after COS than MIS at all three levels of the paraspinal muscle, except for the multifidus muscle at the cranial adjacent level. However, the only significant MFI change difference between the COS and MIS groups was found at the caudal adjacent level of the erector spinae muscle (54.48 ± 37.95% vs. 23.61 ± 31.59%, P = 0.016).
Postoperative MFI change in the multifidus and erector spinae muscles at the operated level (a), cranial adjacent level (b), and caudal adjacent level (c) at follow-up MRI 1 year after operation. There was a trend of more severe fatty infiltration in the COS group. A significant MFI change difference between the two methods was only noted in the erector spinae muscle at the caudal adjacent level.
The average improvement of DSCSA postoperatively was significantly greater in the COS group (128.15 ± 39.83 mm2) compared to the MIS group (78.15 ± 38.5 mm2) (P = 0.0005) (Fig. 6). All functional scores (VAS, ODI. and JOA) were improved 1 year postoperatively; however, there were no significant differences between the two groups (Table 1).
Discussion
Posterior spinal fusion surgery is effective for treating degenerative spine pathologies with spinal stenosis and instability. In COS, the paraspinal muscles need to be deflected from the spinous processes, and this can cause muscle denervation, ischemic, and progressive muscle atrophy2,7,8,9. A number of MIS methods have been developed to avoid extensive muscle dissection, and have become popular.
Several studies have compared postoperative paraspinal muscle degeneration occurring after COS and MIS using MRI or computed tomography (CT) (Table 2)1,3,9,10,11,12,13,14,15. Mori et al. evaluated muscle damage from COS using the spinous process-splitting approach for decompression, and found significant postoperative multifidus muscle atrophy at the operated and caudal adjacent levels15. Min et al. reported less multifidus muscle degeneration following MIS than COS surgery by measuring the average cross-sectional area and fat infiltration of muscle at the L1–5 levels1. Stevens et al. reported less edema and atrophy of the multifidus muscle in patients that received MIS as compared to those that received COS at 6 months postoperatively13.
MRI provides a safe method for quantitative and qualitative evaluation of muscle without ionizing radiation. The only disadvantage of MRI for postoperative paraspinal muscle evaluation is artifacts due to the metallic implants. Most studies evaluating the paraspinal muscle postoperatively have used a cross-sectional area measurement. But we found that the metallic artifacts due to pedicle screws and rods on MRI result in poor inter-observer reliability of cross-sectional area measurement. For this reason, we used the MFI to reflect the degree of fatty infiltration in the paraspinal muscles5,6. Increased muscle fatty infiltration reflects decreased muscle quality, and might be a contributing factor of back pain6. Postoperative muscle edema and swelling can be present for up to 6 months after surgery, before it begins to subside13,17. For this reason, we performed the follow-up MRI evaluations at approximately 1 year after surgery, and used T1-weighted images for MFI qualification18.
The results of our study showed a trend of more severe postoperative fatty infiltration in both the multifidus and erector spinae muscle in the COS group than in the MIS group. In addition, the results showed muscular fatty degeneration was more prominent in the paraspinal muscles, consistent with similar studies in the literature. A significantly greater increase of MFI in the erector spinae muscle at caudal adjacent level was found in the COS group. While fatty infiltration of the erector spinae muscle at the operated level and the cranial adjacent level, and of the multifidus muscle at the operated, cranial, and caudal adjacent levels were all greater in the COS group, differences between the COS and MIS group were not significant.
Several studies have shown that fatty infiltration in the paraspinal muscles is more prominent in the lower lumbar segments in asymptomatic persons18,19. Olivier et al. found alteration of the contractile component of the cross-section area in the erector muscle mainly occurs distal to the lumbar operated segment20. In our study, fatty infiltration in postoperative muscle was also more prominent at the caudal adjacent level then the cranial adjacent level. This finding is likely because fatty infiltration of skeletal muscle usually begins at more distal skeletal locations21. As such, we would expect that postoperative muscular fatty atrophy will be earlier detected at lower lumbar levels.
Julio et al. suggested that single-level radiological morphology of the paraspinal muscle was not representative of the whole lumbar spine, and multi-level evaluation was more suitable for research of the entire lumbar musculature22. In this study, we evaluated global postoperative paraspinal muscular change by using the average MFI of L2–S1. We found increased postoperative fatty infiltration in the multifidus and erector spinae muscles in both groups, but a significant MFI change between the two operative methods was only found in the erector spinae muscle.
MIS laminectomy/laminotomy can provide good results of decompression of spinal stenosis as compared with COS23,24. To our knowledge, no study comparing postoperative DSCSA changes between COS and MIS has been published. DSCSA has been reported to be significantly associated with clinical outcomes after surgery for lumbar spinal canal stenosis25. Our evaluation of postoperative DSCSA changes indicated better decompression in the COS group than the MIS group. But all functional scores (VAS, ODI, and JOA) 1 year postoperatively were not different between the two groups. This finding suggests that the degree of decompression may not be the only factor that determines postoperative functional improvement.
There are some limitations to this study. For MFI evaluation, the preoperative ROI corresponding to the postoperative ROI was drawn manually by visualization. This may have resulted in some error; however, there was good inter-observer reliability. This was a single-center study with a small sample size, and only 1-year follow-up. Further studies with larger sample size and long-term follow-up are required to confirm the results.
Conclusions
There was a trend of more severe fatty degeneration of the paraspinal muscles after COS than MIS. A statistical significant MFI difference between the two groups was only noted in the erector spinae muscle at the caudal adjacent level, and L2–5 mean global level. Postoperative muscular fatty infiltration was more prominent (may be more detectable) at the lower lumbar level. Decompression of spinal stenosis was significantly greater in the COS group as determined by DSCSA, but there was no significant difference of functional scores between the two groups at 1-year follow-up.
References
Min, S. H., Kim, M. H., Seo, J. B., Lee, J. Y. & Lee, D. H. The quantitative analysis of back muscle degeneration after posterior lumbar fusion: Comparison of minimally invasive and conventional open surgery. Asian Spine J. 3, 89–95. https://doi.org/10.4184/asj.2009.3.2.89 (2009).
Suwa, H. et al. Postoperative changes in paraspinal muscle thickness after various lumbar back surgery procedures. Neurol. Med. Chir. (Tokyo) 40, 151–154 (2000) (discussion 154–155).
Fan, S. et al. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: Minimally invasive procedure versus conventional open approach. Eur. Spine J. 19, 316–324. https://doi.org/10.1007/s00586-009-1191-6 (2010).
Mayer, H. M. The ALIF concept. Eur. Spine J. 9(Suppl 1), S35-43 (2000).
Hyun, S. J., Kim, Y. J. & Rhim, S. C. Patients with proximal junctional kyphosis after stopping at thoracolumbar junction have lower muscularity, fatty degeneration at the thoracolumbar area. Spine J. 16, 1095–1101. https://doi.org/10.1016/j.spinee.2016.05.008 (2016).
D’Hooge, R. et al. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man. Ther. 17, 584–588. https://doi.org/10.1016/j.math.2012.06.007 (2012).
Elliott, J. M. et al. Magnetic resonance imaging analysis of the upper cervical spine extensor musculature in an asymptomatic cohort: An index of fat within muscle. Clin. Radiol. 60, 355–363. https://doi.org/10.1016/j.crad.2004.08.013 (2005).
Fritzell, P., Hagg, O., Wessberg, P., Nordwall, A. & Swedish Lumbar Spine Study, G. Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976) 27, 1131–1141 (2002).
Kim, D. Y., Lee, S. H., Chung, S. K. & Lee, H. Y. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976) 30, 123–129 (2005).
Pourtaheri, S. et al. Paraspinal muscle atrophy after lumbar spine surgery. Orthopedics 39, e209-214. https://doi.org/10.3928/01477447-20160129-07 (2016).
Bresnahan, L. E. et al. Assessment of paraspinal muscle cross-sectional area after lumbar decompression: Minimally invasive versus open approaches. Clin. Spine Surg. 30, E162–E168. https://doi.org/10.1097/BSD.0000000000000038 (2017).
Kim, K., Isu, T., Sugawara, A., Matsumoto, R. & Isobe, M. Comparison of the effect of 3 different approaches to the lumbar spinal canal on postoperative paraspinal muscle damage. Surg. Neurol. 69, 109–113. https://doi.org/10.1016/j.surneu.2007.04.021 (2008) (discussion 113).
Stevens, K. J. et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J. Spinal Disord. Tech. 19, 77–86. https://doi.org/10.1097/01.bsd.0000193820.42522.d9 (2006).
Hyun, S. J. et al. Postoperative changes in paraspinal muscle volume: Comparison between paramedian interfascial and midline approaches for lumbar fusion. J. Korean Med. Sci. 22, 646–651. https://doi.org/10.3346/jkms.2007.22.4.646 (2007).
Mori, E. et al. Spinous process-splitting open pedicle screw fusion provides favorable results in patients with low back discomfort and pain compared to conventional open pedicle screw fixation over 1 year after surgery. Eur. Spine J. 21, 745–753. https://doi.org/10.1007/s00586-011-2146-2 (2012).
Tsutsumimoto, T., Shimogata, M., Ohta, H. & Misawa, H. Mini-open versus conventional open posterior lumbar interbody fusion for the treatment of lumbar degenerative spondylolisthesis: comparison of paraspinal muscle damage and slip reduction. Spine (Phila Pa 1976) 34, 1923–1928. https://doi.org/10.1097/BRS.0b013e3181a9d28e (2009).
Gejo, R., Matsui, H., Kawaguchi, Y., Ishihara, H. & Tsuji, H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine (Phila Pa 1976) 24, 1023–1028 (1999).
Crawford, R. J. et al. Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am. J. Neuroradiol. 37, 742–748. https://doi.org/10.3174/ajnr.A4596 (2016).
Lee, S. H., Park, S. W., Kim, Y. B., Nam, T. K. & Lee, Y. S. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J. 17, 81–87. https://doi.org/10.1016/j.spinee.2016.08.001 (2017).
Gille, O. et al. Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine (Phila Pa 1976) 32, 1236–1241. https://doi.org/10.1097/BRS.0b013e31805471fe (2007).
Hamrick, M. W., McGee-Lawrence, M. E. & Frechette, D. M. Fatty infiltration of skeletal muscle: Mechanisms and comparisons with bone marrow adiposity. Front. Endocrinol. (Lausanne) 7, 69. https://doi.org/10.3389/fendo.2016.00069 (2016).
Urrutia, J. et al. Is a single-level measurement of paraspinal muscle fat infiltration and cross-sectional area representative of the entire lumbar spine?. Skelet. Radiol. 47, 939–945. https://doi.org/10.1007/s00256-018-2902-z (2018).
Mobbs, R. J., Li, J., Sivabalan, P., Raley, D. & Rao, P. J. Outcomes after decompressive laminectomy for lumbar spinal stenosis: Comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J. Neurosurg. Spine 21, 179–186. https://doi.org/10.3171/2014.4.SPINE13420 (2014).
Wong, A. P., Smith, Z. A., Lall, R. R., Bresnahan, L. E. & Fessler, R. G. The microendoscopic decompression of lumbar stenosis: A review of the current literature and clinical results. Minim. Invasive Surg. 2012, 325095. https://doi.org/10.1155/2012/325095 (2012).
Mannion, A. F. et al. Dural sac cross-sectional area and morphological grade show significant associations with patient-rated outcome of surgery for lumbar central spinal stenosis. Eur. Spine J. 26, 2552–2564. https://doi.org/10.1007/s00586-017-5280-7 (2017).
Acknowledgements
This work was kindly supported by Grants CRRPG3H0061-2 from Chang Gung Memorial Hospital, Bone and Joint Research Center.
Author information
Authors and Affiliations
Contributions
C.J.F., C.H.C. and C.C.N. contributed to the conception or design of the work. C.H.C. and C.C.N. contributed to drafting the work and revising it critically for important intellectual content. W.C.C. and M.L.L. contributed to interpret and analyse data for the work. C.J.F. and C.C.N. wrote the first draft of the manuscript and all authors contributed to the review, approval of the final manuscript, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, CJ., Chen, WC., Lu, ML. et al. Comparison of paraspinal muscle degeneration and decompression effect between conventional open and minimal invasive approaches for posterior lumbar spine surgery. Sci Rep 10, 14635 (2020). https://doi.org/10.1038/s41598-020-71515-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71515-8
This article is cited by
-
Correlation between surgical segment mobility and paravertebral muscle fatty infiltration of upper adjacent segment in single-segment LDD patients: retrospective study at a minimum 2 years’ follow-up
BMC Musculoskeletal Disorders (2023)
-
Comparison between OLIF and MISTLIF in degenerative lumbar stenosis: an age-, sex-, and segment-matched cohort study
Scientific Reports (2023)
-
Oblique lumbar interbody fusion versus minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative disease of the lumbar spine: a systematic review and meta-analysis
Neurosurgical Review (2023)
-
Lyophilized bovine acellular tendon linear fiber material for the reconstruction of attachment structure of paraspinous muscles: an animal in vivo study
Journal of Materials Science: Materials in Medicine (2022)
-
The effect of posterior lumbar dynamic fixation and intervertebral fusion on paraspinal muscles
BMC Musculoskeletal Disorders (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.