Abstract
Deep learning (DL) is an advanced machine learning approach used in diverse areas such as bioinformatics, image analysis, and natural language processing. Here, using brain magnetic resonance imaging (MRI) data obtained at early stages of infarcts, we attempted to develop a convolutional neural network (CNN) to predict the ambulatory outcome of corona radiata infarction at six months after onset. We retrospectively recruited 221 patients with corona radiata infarcts. A favorable outcome of ambulatory function was defined as a functional ambulation category (FAC) score of ≥ 4 (able to walk without a guardian’s assistance), and a poor outcome of ambulatory function was defined as an FAC score of < 4. We used a CNN algorithm. Of the included subjects, 69.7% (n = 154) were assigned randomly to the training set and the remaining 30.3% (n = 67) were assigned to the validation set to measure the model performance. The area under the curve was 0.751 (95% CI 0.649–0.852) for the prediction of ambulatory function with the validation dataset using the CNN model. We demonstrated that a CNN model trained using brain MRIs captured at an early stage after corona radiata infarction could be helpful in predicting long-term ambulatory outcomes.
Similar content being viewed by others
Introduction
Stroke is a leading cause of disability in humans, and ambulatory dysfunction is one of the most severe disabling sequelae1. For stroke patients, the accurate prediction of ambulatory dysfunction is crucial in terms of rehabilitation. Several methods have been proposed to estimate the ambulatory outcomes in stroke patients based on clinical findings, imaging studies [magnetic resonance imaging (MRI) and computed tomography (CT)], and electrophysiological studies2,3,4,5. Recently, various methods such as diffusion tensor imaging and functional magnetic resonance have been employed to increase the motor-function prediction accuracy6,7.
Machine learning (ML) entails computer algorithms that can automatically learn from data without requiring explicit programming8. Recently, ML techniques have been applied to diverse areas such as bioinformatics, image analysis, and natural language processing9. The use of ML helps overcome the limitations of existing techniques and has enabled breakthroughs in several areas. Some previous studies used ML for predicting motor function after a stroke with clinical data as input variables10,11,12. However, an excessively large number of input variables were used; moreover, the clinical data obtained in various hospitals were different. Therefore, it was not possible to use an ML algorithm designed for one hospital in another hospital. Imaging studies, such as brain MRI and CT, could performed for all stroke patients in all clinics. Consequently, an algorithm that employs brain imaging results as input variables can be widely used.
The deep learning (DL) technique is one of the advanced ML approaches. Particularly, it constructs artificial neural networks with structures and functions similar to those of the human brain by using a large number of hidden layers13. The DL technique can outperform traditional ML techniques and can learn from unstructured and perceptual data such as images and languages. A convolutional neural network (CNN) is a representative DL model that has a significant advantage in imaging recognition and classification14.
In this study, using brain MRI data obtained at early stages of corona radiata infarcts, we developed a CNN to predict the ambulatory outcome of corona radiata infarction at six months after onset.
Results
The receiver operating characteristic curve analysis and AUC calculation were performed using scikit-learn. Of the 221 patients, 101 (45.7%) showed a favorable outcome of ambulatory function at the six-month follow-up after stroke onset and 120 (54.3%) showed a poor outcome.
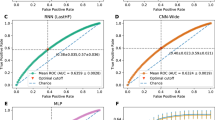
In the prediction of ambulatory function with the validation dataset using the CNN model, the AUC was 0.751 (95% CI 0.649–0.852) (Fig. 1).
Discussion
In the present study, we developed a CNN model for predicting the ambulatory outcome six months after corona radiata infarction using MR images as input data.
In our study, the AUC of the model, evaluated with the validation dataset, was 0.751 with regard to predicting the ambulatory function at six months after the onset of corona radiata infarction. Considering that AUCs ranging from 0.7 to 0.8 are generally considered acceptable15, the proposed CNN model trained using brain MRI input data obtained at an early stage of cerebral infarction can be helpful for clinicians in predicting long-term ambulatory outcomes.
A deep neural network (DNN) is characterized by a multilayer perceptron with multiple hidden layers or a feedforward neural network, which provides greater ability than a traditional shallow neural network13. A CNN is representative of a DNN model; it receives multiple channels of two-dimensional data as input and transforms them repeatedly using convolution and pooling operations14. These processes allow the extraction of valuable features from the input data. CNNs are widely used in image processing and pattern recognition. In prior reports on the prediction of the motor outcome of cerebral infarction using images, infarct volume and leukoaraiosis were reported to be correlated with post-infarction motor outcomes16,17. Apart from these factors, damage to the corticospinal tract (CST) is also known to be a decisive factor for motor prognosis. Therefore, for the accurate prediction of motor outcomes, the state of the CST should also be evaluated. To evaluate the state of the CST, diffusion tensor tractography is used7. However, this method is limited because of false negatives and positives18. We proposed that a CNN model could determine whether cerebral infarcts involved areas through which the CST passed. In addition, during the development of the algorithm, we determined that the infarct volume and degree of leukoaraiosis should be considered for predicting the motor outcome. However, owing to the nature of the DNN, we could not know which factors the algorithm considered (and the manner in which they were weighted) for predicting the ambulatory outcome after corona radiata infarction.
To date, several studies have evaluated the application of machine learning to predict the motor outcome after a stroke10,11,12. Heo et al. predicted the modified Rankin Scale score at 3 months after ischemic stroke using deep neural network, logistic regression, and random forest with 2604 acute ischemic stroke subjects. They used 38 variables including patient demographics, initial National Institutes of Health Stroke Scale scores, stoke subtypes, and time from onset to admission as inputs. The AUCs were 0.888, 0.849, and 0.857, respectively10. Sale et al. studied the predictability of improving motor function after rehabilitation treatment from the early stages of stroke. They used 55 patients' data collected at the time of admission to the Department of Rehabilitation Medicine and discharge, and predicted the Barthel Index and functional independence measure score with a linear support vector machine regression model. All output results and the actual measured results showed a good correlation of 0.75–0.8112. Lin et al. constructed a prognostic model of functional outcome using data from 313 patients with stroke. Various functional measurement outcomes at early stage after storke, such as modified Rankin Scale and Barthel Index scores, gait speed, and results of Mini-Mental State Examination, were used as inputs. The Barthel index status at discharge was predicted. They utilized logistic regression, support vector machine, and random forest models, and AUCs were 0.792, 0.774, and 0.792, respectively11. Although the AUCs of the previous studies tend to be higher than that of our study, they used clinical data as input variables for predicting the motor prognosis, without using image data. For developing algorithms using clinical data, an excessively large number of variables are necessary. In addition, each hospital collects different types of clinical data. Therefore, in clinical practice, it would be difficult to globally use a specific model developed using clinical data as input variables. In contrast to previous models, we used only MR images to develop the model. Brain MRI is used for diagnosing cerebral infarction in every hospital; therefore, we believe that a DL algorithm developed using brain MR images can be practically used in clinical practice. To the best of our knowledge, our study is the first to show the possibility of using DL algorithms trained using MR images for predicting the ambulatory outcomes after a stroke.
Our study was limited in that we used a small number of brain MRI data for training the CNN model. We think that, if a larger number of input MRI data are used, the accuracy of the model will increase. In addition, we believe that developing a model that integrates MR images and patients’ clinical data would be helpful for increasing the accuracy of the model for predicting the ambulatory outcomes after corona radiata infarction.
In conclusion, we demonstrated that a CNN model trained using brain MRIs captured at an early stage after corona radiata infarction could be helpful for predicting long-term ambulatory outcomes. The accuracy of the model was acceptable, but not considerably high. Therefore, it should be used as a supplementary tool for clinicians to predict long-term ambulatory outcomes. In addition, we believe that the combined use of clinical data with brain MRI as input data may be helpful for increasing the accuracy of the model for predicting the ambulatory outcomes after corona radiata infarction.
Materials and methods
Subjects
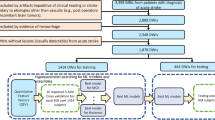
We retrospectively recruited 221 consecutive patients with corona radiata infarcts (mean age = 65.0 ± 11.9, M:F = 115:106) who underwent stroke rehabilitation in a single university hospital from January 2003 to January 2020 for this study (Table 1). The inclusion criteria were as follows: (1) first-ever stroke; (2) age over 20 years; (3) hemiplegia or hemiparesis following corona radiata infarction; (4) brain MRI conducted within 30 days after onset; (4) functional ambulatory category (FAC) checked six months after stroke onset; and (5) absence of other serious medical complications, such as pneumonia or cardiac problems, during the period from onset to final evaluation. The study protocol was approved by the institutional research board of Yeungnam university hospital (No. 2019-10-008). The institutional research board waived the need for informed consent for this study since we used de-identified retrospective data. This study followed Helsinki Declarations.
Images used for deep learning (input variables)
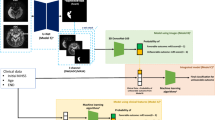
Three T2-axial consecutive brain MR images obtained from each patient were used in our study. The images at the levels of the body of the lateral ventricle were collected, such that corona radiata fibers passing above the internal capsule could be observed (Fig. 2). The MR images captured on the day closest to the date of transfer to the rehabilitation department, within 30 days after infarction onset, were used for the analysis.
Motor outcome at six months (output variables)
We used the FAC score as the output data. The FAC is used for quantifying ambulatory function (walking ability), and it is based on the characterization of the levels of assistance required during a 15 m walk19. The FAC is categorized as follows: 0: nonambulatory, 1: continuous support from one person necessary, 2: intermittent support from one person necessary, 3: requirement of verbal supervision only, 4: assistance required on stairs and uneven surfaces, and 5: can walk independently anywhere. We categorized the FAC at six months after the onset as follows: favorable outcomes for lower limbs: FAC score ≥ 4 (able to walk without a guardian’s assistance) and poor outcomes for lower limbs: FAC score < 4.
Deep learning algorithms
We used a CNN algorithm implemented using the Python programming language. Tensorflow 2.3, the Keras framework, and scikit-learn toolkit 0.23.2 were used to train the ML model. The details of the model and its performance are described in Table 2. The classifier with a majority voting algorithm was employed to predict the motor outcomes of individual patients. For example, when the predictions corresponding to two images were favorable outcomes and that corresponding to a third image was a poor outcome, the final decision for the patient was a favorable outcome.
Among the included subjects, 69.7% (n = 154) were selected randomly for the training set and the remaining 30.3% (n = 67) were assigned to the validation set to measure the model performance.
Statistical analysis
A receiver operating characteristic curve analysis was performed, and the area under the curve (AUC) was calculated using scikit-learn. The confidence interval for the AUC was calculated using the approach used by DeLong et al.20.
Data availability
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
References
Belda-Lois, J. M. et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J. Neuroeng. Rehabil. 8, 66 (2011).
Chang, M. C., Do, K. H. & Chun, M. H. Prediction of lower limb motor outcomes based on transcranial magnetic stimulation findings in patients with an infarct of the anterior cerebral artery. Somatosens. Mot. Res. 32, 249–253 (2015).
Jones, P. S. et al. Does stroke location predict walk speed response to gait rehabilitation?. Hum. Brain. Mapp. 37, 689–703 (2016).
Kim, B. R., Moon, W. J., Kim, H., Jung, E. & Lee, J. Transcranial magnetic stimulation and diffusion tensor tractography for evaluating ambulation after stroke. J. Stroke 18, 220–226 (2016).
Piron, L., Piccione, F., Tonin, P. & Dam, M. Clinical correlation between motor evoked potentials and gait recovery in poststroke patients. Arch. Phys. Med. Rehabil. 86, 1874–1878 (2005).
Dobkin, B. H., Firestine, A., West, M., Saremi, K. & Woods, R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage 23, 370–381 (2004).
Jang, S. H., Choi, B. Y., Chang, C. H., Kim, S. H. & Chang, M. C. Prediction of motor outcome based on diffusion tensor tractography findings in thalamic hemorrhage. Int. J. Neurosci. 123, 233–239 (2013).
Deo, R. C. Machine learning in medicine. Circulation 132, 1920–1930 (2015).
Jiang, F. et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc. Neurol. 2, 230–243 (2017).
Heo, J. et al. Machine learning-based model for prediction of outcomes in acute stroke. Stroke 50, 1263–1265 (2019).
Lin, W. Y. et al. Predicting post-stroke activities of daily living through a machine learning-based approach on initiating rehabilitation. Int. J. Med. Inform. 111, 159–164 (2018).
Sale, P. et al. Predicting motor and cognitive improvement through machine learning algorithm in human subject that underwent a rehabilitation treatment in the early stage of stroke. J. Stroke Cerebrovasc. Dis. 27, 2962–2972 (2018).
Abiodun, O. I. et al. State-of-the-art in artificial neural network applications: a survey. Heliyon. 4, e00938 (2018).
Yamashita, R., Nishio, M., Do, R. K. G. & Togashi, K. Convolutional neural networks: an overview and application in radiology. Insights Imaging 9, 611–629 (2018).
Mandrekar, J. N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 5, 1315–1316 (2010).
Liu, Y. et al. The degree of leukoaraiosis predicts clinical outcomes and prognosis in patients with middle cerebral artery occlusion after intravenous thrombolysis. Brain Res. 1681, 28–33 (2018).
Schiemanck, S. K., Post, M. W., Witkamp, T. D., Kappelle, L. J. & Prevo, A. J. Relationship between ischemic lesion volume and functional status in the 2nd week after middle cerebral artery stroke. Neurorehabil. Neural Repair 19, 133–138 (2005).
Calamante, F. The seven deadly sins of measuring brain structural connectivity using diffusion MRI streamlines fibre-tracking. Diagnostics (Basel) 9, 115 (2019).
Cunha, I. T. et al. Performance-based gait tests for acute stroke patients. Am. J. Phys. Med. Rehabil. 81, 848–856 (2002).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1998).
Acknowledgements
None.
Funding
This study was supported by the Medi-Challenger Program funded by the Gyeongsan City and a National Research Foundation of Korea Grant funded by the Korean government (Grant no. NRF-2021R1A2C1013073).
Author information
Authors and Affiliations
Contributions
J.K.K., Y.J.C., H.S., and G.S.C.: study design, data analysis, manuscript writing/editing. M.C.C.: study design, data analysis, manuscript writing/editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.K., Choo, Y.J., Shin, H. et al. Prediction of ambulatory outcome in patients with corona radiata infarction using deep learning. Sci Rep 11, 7989 (2021). https://doi.org/10.1038/s41598-021-87176-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87176-0
This article is cited by
-
Convolutional neural network algorithm trained on lumbar spine radiographs to predict outcomes of transforaminal epidural steroid injection for lumbosacral radicular pain from spinal stenosis
Scientific Reports (2024)
-
Localization of early infarction on non-contrast CT images in acute ischemic stroke with deep learning approach
Scientific Reports (2023)
-
Deep learning algorithm to evaluate cervical spondylotic myelopathy using lateral cervical spine radiograph
BMC Neurology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.