Abstract
The potential reproductive toxic effects of oral TiO2 NPs in adult male rats as well as the possible alleviation of chitosan administration was investigated. Animals were allocated to four groups; the first group received deionized water and was assigned as a control group. In the second group, rats received chitosan at a dose of 5 mg/kg BW/day. The third group was designed for administration of TiO2 NPs at a dose of 150 mg/kg BW/day (1/80 LD50). Rats in the fourth group received both TiO2 NPs and chitosan. After 14 days, TiO2 NPs induced testicular lipid peroxidation as well as oxidative stress. Nano-titanium significantly upregulated genes that encode apoptosis and inflammation in testicular tissue. Moreover, it induced histological alteration in the testicular structure with impairment in spermatogenesis via reduction of PCNA immune-staining. Chitosan administration significantly improved the activities of testicular GPx, SOD, and CAT enzymes. In addition, it significantly down-regulated the relative expressions of pro-apoptotic and pro-inflammatory testicular genes. Chitosan was able to improve the testicular architecture as well as spermatogenesis. The current study revealed the capability of chitosan to ameliorate nano-titanium induced testicular toxicity. Thus, attention should be given to the extensive consumption of nano-titanium particles.
Similar content being viewed by others
Nanomaterials gain advantages since they cross various body barriers for therapeutic applications as well as their integration into many industrial products, including medical devices, commercial products, and electronics. Nevertheless, this technology has some negative health issues in humans and animals1,2. Among nanoparticles, titanium dioxide nanoparticles (TiO2 NPs) are widely used in various oral- and dermal consumed products, therefore the exposure to these nanoparticles is unavoidable. Several studies were established for investigation of the toxic impact of TiO2 NPs on different body organs, particularly those relating to reproductive issues3,4. Reproduction is the way to preserve the species, thus keeping the ecosystem balanced.
Reproductive organs are very sensitive to nanoparticles. It is well documented that TiO2 NPs are able to cross the blood–testes and blood–brain barriers5,6,7. TiO2 NPs have been validated to accumulate in organs and result in toxicity, with inadequate data regarding male reproductive toxicity caused by TiO2 NPs8. TiO2 NPs can cross the blood-testis barrier to reach the testis and accumulate, resulting in testicular lesions, shifts in serum sex hormone levels, and cell apoptosis3,4. Intravenous TiO2 NPs induced testicular dysfunction via reduction in testosterone hormone, induction of oxidative stress and apoptosis with bioaccumulation of TiO2 NPs in testicular cells of mice9.
Chitosan is a natural substance used in medical applications for therapeutic purposes. It is derived from several types of the compound chitin, which is widely found in the outer shells of crustacean species such as shrimp and crabs10. Owing to its unique physical and chemical properties, chitosan gained more interest and was integrated into a wide range of applications in the medical field as an antibacterial agent, a drug carrier, and wound healing11. Chitosan improves the sperm quality of lead acetate-intoxicated rats12.
Reliant on oral LD50 of TiO2 for rats (> 12,000 mg/kg BW; WHO 1969), we investigated the influence of sub-acute oral TiO2 NPs (1/80 LD50) as well as the possible ameliorating effect of chitosan on various testicular biomarkers including oxidative stress/antioxidant parameters, inflammation, and apoptosis in the testes of adult rats.
Results
Characterization of TiO2 NPs
Titanium dioxide (TiO2) powders were prepared by ball milling; the crystalline powder of TiO2 was confirmed by X-ray-diffraction (XRD) also, the size of TiO2 nanoparticles was average 50–55 nm (Fig. 1). a HRSEM images of a TiO2 nanoparticle showed structure, distribution, and size at 100 nm (Fig. 2A) and 1 µm Scale (Fig. 2B). Fourier-transform infrared (FTIR) spectra for Tio2 showed the peaks only corresponding to TiO2 at 510 and 680 cm−1 (Fig. 3).
Index weight of testis
Rats that received TiO2 NPs showed no alteration in the index weight of their testes (p > 0.05) (Table 1).
Serum testosterone level
Nano titanium particles significantly reduced serum levels of testosterone hormone compared to the control group (p < 0.05). Chitosan showed no obvious effect on testosterone hormone either alone or combined with TiO2 NPs (Fig. 4).
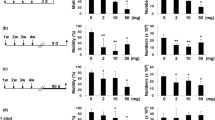
Testicular oxidative/antioxidant biomarkers (Fig. 5)
Effects of TiO2 NPs and/or chitosan on testicular oxidative/antioxidant markers. data are presented as means ± SEM. data are presented as means ± SEM. Data are expressed as mean ± standard error of the mean (n = 5). Means in the same row with different superscripts are significantly different (p < 0.05). MDA (malondialdehyde); GSH (Reduced glutathione); GST (Glutathione S transferase); GPx (Glutathione Peroxidase) SOD (superoxide dismutase) and CAT (catalase).
After 14 days, nano titanium particles significantly increased testicular MDA levels compared to the control group. Chitosan treatment significantly decreased MDA levels compared to the TiO2 NPs group (p < 0.05).
Glutathione (GSH) levels were markedly reduced in the TiO2 NPs group compared to the control group. Chitosan significantly alleviated the TiO2 NPs-induced reduction of GSH. The activity of GST enzyme was seriously diminished in TiO2 NPs intoxicated rats compared with the control group with a significant improvement in testicular activity of GST in TiO2 NPs + Ch group compared to single administration of TiO2 NPs. Compared to the control group, the activity of testicular GPx enzyme was markedly reduced in TiO2 NPs—intoxicated rats in both TiO2 NPs and TiO2 NPs + Ch groups without significant variation between them.
Nano-titanium particles severely reduced the activity of testicular SOD enzyme compared to the control group (p < 0.05). Chitosan significantly mitigated the TiO2 NPs-induced reduction in SOD activity in the TiO2 NPs + Ch group. The activity of CAT enzyme was enormously declined in the TiO2 NPs group compared to the control one. Chitosan significantly increased the testicular activity of CAT in both, chitosan and TiO2 NPs + Ch groups compared to the control and the TiO2 NPs groups, respectively (p < 0.05).
Relative expression of pro-apoptotic genes (Fig. 6A)
The relative expressions of caspase 3 and BAX were immensely up-regulated in the TiO2 NPs and TiO2 NPs + Ch groups compared to the control group (p < 0.05) with a significant alleviating effect of chitosan in the TiO2 NPs + Ch group.
Relative expression of pro-inflammatory genes (Fig. 6B)
Chitosan showed significant downregulation of testicular genes encode inflammation, TNFα and IL-1β, compared to the control group (p < 0.05). Nano-titanium particles extremely upregulated the testicular mRNA expression of both TNFα and IL-1β in the TiO2 NPs group compared to the control one (p < 0.05). Chitosan treatment significantly downregulated their relative expressions in the TiO2 NPs + Ch group compared to single administration of TiO2 NPs.
Histological analysis (H & E stain)
As shown in Fig. 5, histological examination of the control group (Fig. 7a1–a3) showed that the testicular parenchyma was studded with numerous normal seminiferous tubules that were separated from the interstitial tissues (Leydig cells and blood capillaries) by a well-defined basal lamina and flat myoid cells. The tubules are lined with 4–8 layers of closely and orderly arranged germinal epithelium (spermatogonia, primary spermatocytes, and spermatids) and Sertoli cells. The histological structure of the testes in the chitosan group (Fig. 7b1–b3) was almost similar to that of the control group (Fig. 7a1–a3). However, in the chitosan group, the seminiferous tubules appeared overcrowded with the germinal epithelium and the interstitial tissues were densely packed with blood capillaries. In the TiO2 NPs group, a significant reduction was observed in the number of normal seminiferous tubules with intact germinal epithelium (p < 0.05) compared to the control group (Fig. 7c1–c3). Various degrees of germinal epithelium degeneration were detected, ranging from germinal epithelium disorganization, sloughing, detachment, and vacuolization. Moreover, some tubules are irregular, atrophied, and empty of their lining germinal epithelium. Although the interstitial blood vessels were markedly congested and large areas of interstitial tissues appeared vacuolated and covered with pale inflammatory exudates, the nuclei of Leydig cells appeared normal. In the TiO2 NPs + Ch group (Fig. 7d1–d3), the mean percentage of preserved seminiferous tubules was significantly increased (p < 0.05) compared to the TiO2 NPs group (Fig. 7A).
Photomicrograph of a section of rat's testis stained with H&E in different groups: (a1–a3) control group, (b1–b3) chitosan group, (c1–c3) TIO2NPs and (d1–d3) TIO2NPs+Ch group, showing seminiferous tubules (black thin arrow), interstitial tissue (black thick arrow), lumen of seminiferous tubules(asterisk), myoepithelial cells (blue vertical arrow), spermatogonia (blue thin arrow), primary spermatocytes (blue corrugated arrow), early spermatid (inside blue circle), mature sperm (tailed blue arrow), hyphae of sperm (thick blue arrow), Leydig cells (blue arrow head), Sertoli cells (curved blue arrow), interstitial blood vessels (blue asterisk), seminiferous tubules with disorganized epithelium (black corrugated arrow), shrinkage seminiferous tubules (black vertical arrow), seminiferous tubules with sloughed and necrotic epithelium (black tailed arrow), congested interstitial blood vessels (white arrow). (A): % of normal seminiferous tubules, data are expressed as Mean ± SEM. The different letters (a, b, c) indicate significant difference between experimental groups.
Spermatogenesis assessment (Fig. 8)
Photomicrograph of a section of rat's testis stained with H&E showing Spermatogenesis assessment of experimental groups (a1–a4) control group, (b1–b4) chitosan group, (c1–c4) TIO2 NPs and (d1–d4) TIO2NPs + Ch group. (A): Johnson scores of the experimental group, data are expressed as Mean ± SEM. The different letters indicate significant difference (p < 0.05) between experimental groups.
Normal spermatogenesis was observed in seminiferous tubules in the control (Fig. 8a1–a4,A) and chitosan (Fig. 8b1–b4,A) groups, without a significant difference in the mean Johnsen's score between them. In the TiO2 NPs group, the mean Johnsen’s score was significantly decreased than in the control group, and seminiferous tubules showed maturation arrest (Fig. 8c1–c4,A). In TiO2 NPs + Ch group, the majority of tubules achieved a normalized Johnsen's score, with the exception of a small number of tubules with a low score, but the mean score increased significantly in comparison to the TiO2 NPs group (Fig. 8d1–d4,A).
Caspase-3 Immunohistochemical determination (Fig. 9A)
Photomicrograph of Enzyme immunohisto-chemical staining of paraffin sections from a testicular tissue for 1. caspase-3 immune expression (a, b, c and d) showing:only some hyphae of mature sperms in control (a) and chitosan (b) have positive reaction for caspase (arrow), most of seminiferous epithelium in TIO2NPs group (c) have positive reaction for caspase (arrow head). In TIO2NPs + Chitosan group, some hyphae (arrow) and few spermatocytes (arrow head) have positive reactions for caspase 2. PCNA immune expression (e, f, g, h) showing the PCNA expression was detected in the spermatogonia in all groups (arrow head) but in chitosan group was also detected in primary spermatocytes (arrow). (e) represent caspase-3 immune expression density, data are expressed as Mean ± SEM. The different letters (a, b, c) indicate significant difference between experimental groups. (A, B) represent caspase-3 and PCNA immune expression density respectively, data are expressed as Mean ± SEM. The different letters indicate significant difference (P < 0.05) between experimental groups.
The density of caspase-3 staining in both the control (Fig. 9a) and chitosan groups (Fig. 9b) showed negligible variation. In the TiO2 NPs group, the caspase-3 staining density was significantly increased compared to the control group (Fig. 7c). In the TiO2 NPs + Ch group, caspase-3 expression was significantly decreased (Fig. 9d) compared to the TiO2 NPs group.
PCNA Immunohistochemical determination (Fig. 9B)
Expression of PCNA in the control group was quite evident in the nuclei of the spermatogonia (Fig. 9e). Chitosan administration significantly increased the expression of PCNA and was apparently detected in the spermatogonia and primary spermatocytes (Fig. 9f). The expression of PCNA was significantly decreased after TiO2 NPs administration (Fig. 9g). In the TiO2 NPs + Ch group, the expression of PCNA was significantly increased (Fig. 9h) compared to the TiO2 NPs group and approached the PCNA expression in the control group (Fig. 9B).
Discussion
The aim of the present work was to investigate the potential ability of nano-titanium to induce testicular damage as well as the possible mitigation by chitosan administration.
In the current study, neither nano-titanium nor chitosan affected the index weight of the testes. Likewise, no significance was reported in either body weights or testicular weights in male mice exposed to TiO2 NPs at doses up to 100 mg/kg BW for 28 days13,14. Mice exposed orally to TiO2 NPs at doses of 10, 50 and 250 mg/kg BW from 28 to 70th postnatal day suffered reductions in the body weight gain solely at the highest dose without significant difference in absolute or relative weights of testes15. A significant reduction in testicular weight was observed in mice exposed to 300 mg/kg BW of TiO2 NPs for 35 days without alteration in body weights13,14. Meena et al. 20159 observed a significant decrease in the average coefficients of testis at the higher dose of TiO2 NPs (50 mg/kg BW for 30 days), whereas, lower doses of TiO2 NPs (5 and 25 mg/kg BW) did not show a significant effect. The reduction in body weight and testicular mass with rising doses of nano-titanium might be attributed to excess accumulation of TiO2 NPs in testes3. This phenomenon showed that the accumulation of titanium in the organs was closely related to the organ-to-body-weight ratio9. Thus, the dose and the exposure time were among the factors that influenced body and organ weights.
After 14 days of exposure time, TiO2 NPs significantly reduced serum levels of testosterone hormone without obvious action of chitosan. In consistency, TiO2 NPs at doses of 50 or 300 mg/kg BW for 30–35 days significantly decreased serum testosterone levels in rats and mice9,16,17. Serum levels of testosterone were significantly reduced after postnatal exposure of mice to 50 and 250 mg/kg/day nano-TiO215. Intragastric TiO2 NPs for 90 consecutive days passed through the blood–testis barrier and accumulated in the testis, with subsequent testicular lesions, sperm malformations, and alterations in serum sex hormone levels3. Testosterone was produced in Leydig cells and in supported Sertoli cells and was required for the attachment of round spermatids to Sertoli cells18. Low levels of testosterone might suppress spermatogenesis and cause dysfunction of Sertoli cells9,19,20. This reduction may contribute to changes in testis histology and spermatogenesis.
Chitosan nanoparticles at a dose of 280 mg/kg BW for 45 consecutive days attenuated the reduction in testosterone induced by hydroxyapatite nanoparticles21. Pretreatment with chitosan nanoparticles (60 mg/kg BW) ameliorated the testicular level of testosterone significantly as compared to the potassium dichromate exposed group21. Following 30 successive days, chitosan nanoparticles at a dose of 280 mg/kg in combination with selenium significantly increased serum levels of testosterone in diclofenac–sodium intoxicated rats22. In the present work, chitosan might require more exposure time and/or a higher dose to achieve a significant attenuation of the hormonal reduction induced by nano-titanium particles.
In the present study, the testicular level of MDA was obviously increased in the TiO2 NPs group, which is compatible with previous studies that were previously established23. MDA content serves as an indicator of the extent of lipid peroxidation and is an indirect reflection of the extent of cell damage14. MDA is the decomposition product of polyunsaturated fatty acids, and its increase is a result of significant accumulation under high antioxidant stress14. Carboxy methylated chitosan significantly reduced testicular lipid peroxidation in the TiO2 NPs + Ch group compared to the TiO2 NPs group, suggesting the capability of chitosan as a natural biomaterial to alleviate the harm induced by nano titanium particles.
Testicular antioxidant system comprises enzymatic and non-enzymatic constituents. The enzymatic components include SOD, GST and GPx enzymes. The defense mechanism involves conversion of superoxide anion to hydrogen peroxide that is eliminated by CAT or GPx. While GST facilitates conjugation of glutathione with xenobiotic agents for their excretions as a detoxification process24. Nano-titanium particles adversely affected the testicular antioxidant system through reductions in GST, GPx, SOD and CAT activities. Similarly, testicular SOD activity was decreased in mice exposed to 100 mg/kg BW TiO2 NPs for 28 days14. Intravenous TiO2 NPs for 30 days significantly decreased the SOD and GPx activities at 25 and 50 mg/kg BW with an increase in CAT activity in Wister rats9. At a higher dose (300 mg/kg), TiO2 NPs enhanced oxidative stress via increasing testicular levels of MDA and reducing testicular levels of SOD, CAT, and GSH16. Similar outcomes were reported at the cellular level using rat, human, or prepubertal porcine primary cultured Sertoli cells25,26. Nano-titanium particles stimulated production of reactive oxygen species in mouse testes after a 60-day exposure period with a subsequent alteration in the activities of spermatogenesis-dependent enzymes27. Thus, oxidative stress and lipid peroxidation might be attributed to the production of free radicals in the testicular tissues and are vital mechanistic paradigms to explain the toxic effects of TiO2 NPs.
Chitosan was able to ameliorate testicular oxidative stress induced by TiO2 NPs, with a significant increase in CAT activity compared to the control group, confirming its antioxidant properties.
Chitosan increased GSH activity and exhibited antioxidant properties by preventing the decrease in CAT and GSH levels caused by sodium fluoride28. In addition, nano-chitosan with hydroxyapatite nanoparticles increased the activities of GPx, GST, CAT, SOD, TAC, and GSH in male rats21. Chitosan-NPs reversed the diclofenac–sodium mediated decrease in testicular antioxidant markers, SOD and CAT22. In accordance, Chitosan significantly lowered lead-induced testicular oxidative stress29. The pretreatment with nano chitosan (60 mg/kg) significantly decreased MDA levels and increased the activity of GPx levels23. Chitosan has a potent antioxidant activity and free radical scavenger potential, which can decrease the lipid peroxidation (MDA level) and increase the antioxidant defense system, which protects against free radical attack with subsequent nullifying of TiO2 NPs-induced testicular damage.
Regarding the relationship between antioxidant defense enzymes and apoptotic factors. Glutathione and glutathione-related enzymes are decreased in both their levels and activities after the incidence of apoptosis30, as the depletion of GSH is critical for cellular death31. Moreover, GST is an enzyme that is required for the protection against DNA damage induced by apoptosis, where exhaustion of GST activity can be found due to the incidence of apoptosis. Superoxide dismutase is an enzyme that is associated with a defense mechanism against free radicals, found to decrease in the case of prostate carcinoma32, and found to be effective when supplemented to decrease apoptosis. The downregulation of catalase as well as the decrease in its activity might inhibit the process of apoptosis33; due to overproduction of hydrogen peroxide. The overexpression of both catalase and Cu/Zn superoxide dismutase decreased the incidence of apoptosis by 40% due to the decrease in caspase 934. In the current study, the administration of TiO2 can result in the propagation of reactive oxygen species that induce the incidence of apoptosis in testicular tissue as a result of the increase of the degree of lipid peroxidation35. Moreover, proinflammatory cytokines such as TNF-alpha can contribute to the induction of apoptosis through stimulation of both c-ABL and p73 pathways during the degradation of retinoblastoma protein36.
In the present work, short term oral exposure to TiO2 NPs extremely induced testicular inflammation via upregulation of mRNA expressions of TNFα and IL-1β genes with a significant anti-inflammatory action of chitosan. In primary cultured rat Sertoli cells, TiO2 NPs induced an inflammatory response via increasing the expression of IL-1β, TNF-α, IFN-γ, and IL-10 in a concentration-dependent manner37. Inflammatory mediators present in the normal testis, such as interleukins and TNFα participate in the regulation of spermatogenesis;38, therefore the disruption in their expression could perturb normal spermatogenesis. In the current study, TiO2 NPs significantly impaired spermatogenesis, evidenced by the reduction that was reported in Johnsen's score.
Our present work showed that the relative expression of both BAX and Caspase 3 was enormously up-regulated in the TiO2 NPs exposed group. Likewise, the expression levels of testicular caspase-3, Nrbp2, and cytochrome c and their proteins were significantly increased in mice exposed to oral TiO2 NPs (2.5, 5 and 10 mg/kg BW) for 90 days in a dose-dependent manner39. Significant upregulation of testicular BAX gene expression was detected in rats receiving oral 300 mg/kg BW/day of TiO2 NPs for 30 days16. Intravenous administration of 50 mg/kg BW/week for 30 days of TiO2 NPs significantly activated testicular caspase 3 in exposed rats with a significant reduction in total sperm count suggesting that apoptosis is considered to be involved in the impairment of spermatogenesis and the seminiferous tubules9. The pro-apoptotic member of Bcl-2 protein family, BAX, stimulates the mitochondrial pathway, whereas caspase-3 is activated in the apoptotic cell by extrinsic (death ligand) and intrinsic (mitochondrial) pathways40. Up-regulation of both BAX and caspase 3 suggests induction of testicular apoptosis via the mitochondrial pathway. A positive correlation was reported between ROS levels and apoptosis in testicular cells. Therefore, oxidative stress encourages apoptotic processes9,24. Additionally, the upregulation of the proinflammatory cytokines, IL-1β and TNFα, might have a crucial role in inducing testicular apoptosis via regulating Bcl2 family protein expression41.
In the current study, chitosan displayed a testicular anti-apoptotic effect in TiO2 NPs + Ch group with respect to the TiO2 NPs-intoxicated group. Pretreatment of mice with chitosan nanoparticles significantly decreased potassium dichromate-induced elevation in testicular contents of caspase 342. Oral chitosan nanoparticles at a dose of 600 mg/kg BW for 11 days could protect rat testis from oxidative damage and apoptosis prompted by lead acetate through reducing MDA levels, caspase 3 mRNA expression and, in addition, increasing levels of SOD and GPx43.
The present results were confirmed by the histological and morphometric outcomes, including a significant reduction in the number of seminiferous tubules with irregular and atrophied tubules and marked germinal epithelium degeneration. In consistency, in rats exposed to 50 mg/kg TiO2 NPs, disorganization and disruption in some seminiferous tubules were observed9. Numerous histological alterations were reported in rats exposed to 10 mg/kg of TiO2 NPs for 90 days, including irregular arrangement of Sertoli cells in the seminiferous tubules, Sertoli cell apoptosis, necrosis of the seminiferous tubules, decreased thickness of the germinative layer and vacuolation9. No histological changes were observed in the testes of mice exposed to 10 mg/kg/day nano-TiO2. Whereas, at higher doses (50 and 250 mg/kg/day), seminiferous tubules showed vacuoles with decreased layers of spermatogenic cells in mice that received the high-dose (250 mg/kg/day) of nano-TiO215. Nano TiO2 particles (50 mg/kg) significant histological alterations in seminiferous tubules, including reduction in their diameter, epithelial vacuolization, sloughing, detachment, and atrophy with abnormal spermatogenesis and significant decline in the Johnsen score17. Significant modifications in the testicular morphology of TiO2 NP-treated rats might be the result of free reactive radicals and subsequent lipid peroxidation16.
Proliferating cell nuclear antigen (PCNA) is a cell cycle regulatory protein marker that is involved in DNA synthesis and has been linked to cell proliferation44. PCNA immune-expression in the testis is used as a proliferative marker for spermatogenesis estimation45,46. It is considered a rapid, reliable, sensitive, and quantitative approach for determining and detecting early testicular toxicity47,48. Spermatogenesis is a dynamic and synchronized process of maturation of stem spermatogonia into mature spermatozoa, which takes place in the seminiferous tubules49. In the current study, the testicular PCNA expression was mapped using the immunohistochemistry technique. The significant decrease in the PCNA immune-staining with TiO2 NPs highlights the ability of nano-titanium particles to impair spermatogenesis, which is further supported by the histological findings and the significant decline in Johnsen's score. The toxic impact of TiO2 NPs on the male spermatogonia has been previously demonstrated50,51. The reduction in Johnsen's score in the TiO2 NPs group may be attributed to the induction of apoptosis and reduction of the active DNA content in the dividing spermatogonia52. The spermatogenic damage in the TiO2 NPs group was attributed as well to the induction of lipid peroxidation and oxidative stress that had harmful effects on spermatogenesis52.
It is noteworthy that chitosan significantly induced testicular immune-expression of PCNA suggesting its capability to improve spermatogenesis under basal conditions. At the same time, chitosan exerted a potential ameliorative effect counter to that of TiO2 NPs through elevations in the regenerated seminiferous tubules, PCNA immune-staining, and a reduction in Caspase-3 expression in respect to the control group. The constructive effect of chitosan on spermatogenesis was obvious as well in raising Johnsen's score compared to the non-treated TiO2 NPs group.
This work was the first that combined nano-titanium particles with chitosan to study their effects on various reproductive parameters. Briefly, we should highlight the ability of TiO2 NPs to induce testicular dysfunction after short-term of exposure via promotion of inflammation, apoptosis and oxidative stress as well as the reduction in testosterone hormone, the issue that necessitate rising attention for the daily and excessive exposure to oral and occupational nano-titanium particles, and considering its potential toxic impact on reproduction. On the other hand, chitosan was the key to mitigating the adverse effect of TiO2 NPs on testicular functions. In the current work, chitosan achieved its role as an antioxidant, anti-inflammatory, and anti-apoptotic biomaterial. In addition, it improved spermatogenesis as well as CAT activity under normal conditions (without TiO2 NPs exposure), indicating its ability to upgrade testicular function, an issue that needs further investigation.
Materials and methods
This study was conducted at Mansoura University with an approved animal use protocol (R/50) in accordance with the Guiding Principles for the Care and Use of Research Animals, Faculty of Veterinary Medicine, Mansoura University, Egypt.
Chemicals
Titanium dioxide (TiO2) was purchased from Sigma-Aldrich Chemical Co., USA. The titanium dioxide nanoparticles, TiO2 NPs, were prepared at the Nanotechnology Unit, Faculty of Postgraduate Studies in Advanced Sciences, Beni-Suef University, according to the methods described by Farghali et al.46. The size range of TiO2 NPs is less than 60 nm. Solutions of dispersed TiO2 NPs were freshly prepared via ultrasonication for 15 min just before oral administration. Carboxymethyl chitosan (CMC, 10%) was purchased from Xin Luk Biotech, China. A field emission scanning electron microscope, FESEM was used to examine the morphologies of the prepared materials (FEI-Quanta FEG-250 SEM). X-ray diffraction, XRD (PANalytical Empyrean, Netherlands) was used to determine phase identification and crystallinity using CuKa radiation (wavelength 1.54045), accelerating voltage of 40 kV, and current of 35 mA. Raman spectroscopy was performed with a Bruker Senterra Raman Microscope (Bruker Optics Inc., Germany). The modified TiO2 NPs were successfully disseminated into the chitosan matrix, as determined by analysis using scanning electron microscopy (SEM) and atomic force microscopy (AFM), and the roughness of the chitosan-TiO2 nanocomposites was greatly reduced. Additionally, thermogravimetric analysis (TGA) of the thermal characteristics revealed that the chitosan-TiO2 nanocomposites with 0.05 percent TiO2 NPs concentration had the best thermal stability. These analyses also revealed that the chitosan interacted with TiO2 NPs and displayed good compatibility53.
Characterization of TiO2 NPs
Titanium dioxide (TiO2) powders were prepared by ball milling; the crystalline powder of TiO2 was confirmed by X-ray-diffraction (XRD) also, the size of TiO2 nanoparticles was average 50 -55 nm (Fig. 1). a HRSEM images of a TiO2 nanoparticle showed structure, Distribution and Size at 100 Nm (Fig. 2A) and 1 µm Scale Fig. 2B). Fourier-transform infrared (FTIR) spectra for Tio2 Showed the peaks only corresponding to TiO2 at 510 and 680 cm−1 (Fig. 3).
Animals and treatment
Adult male Albino rats (3–4 months old), purchased from MERC lab (Mansoura University) were kept for acclimatization under standard laboratory conditions (temperature of 22–25 °C, 50–60% relative humidity, and 12 h dark/light cycle) for 7 days. Food and water were available ad libitum.
Animals were randomly distributed into four groups (5 rats each). The first group received deionized water and was assigned as a control group. In the second group, animals received chitosan at a dose of 5 mg/kg BW/day according to Wang et al.54. The third group was designed for administration of TiO2 NPs at a dose of 150 mg/kg BW/day (1/80 LD50) according to Azim et al.55. Rats in the fourth group received both TiO2 NPs and chitosan (TiO2 NPs + Ch) (150 and 5 mg/kg BW/day, respectively). Both TiO2 NPs and chitosan were freshly prepared just before administration, following the manufacturers’ instructions. All treatments were given via oral route for 14 consecutive days. On the 15th day, rats were weighed and killed by cervical dislocation. Blood and testes were collected from all groups. Relative testicular weights were calculated using the following equation according to Bearden and Fuquay56.
Serum testosterone level
Serum total testosterone levels were determined following the manufacturer’s instructions of specific kits purchased from Roche-Cobas company (USA; REF. 05200067 190).
Oxidative/antioxidant parameters
The lipid peroxidation marker, malondialdehyde (MDA), was measured according to Draper and Hadley57. Antioxidant defense markers were determined using colorimetric commercial kits [Bio-diagnostic Co, Giza, Egypt]. Reduced glutathione (GSH) concentration was measured colorimetrically using dithionitrobenzoate reagent according to Beutler et al.58. Glutathione S transferase (GST) activity was measured according to Habig et al.59. The activity of superoxide dismutase (SOD) was measured spectrophotometrically according to Nishikimi et al.60.
Real-time PCR
RNA isolation and cDNA synthesis
Testicular tissues were homogenized (100 mg/1 ml) in Trizol™ reagent (Invitrogen, UK) according to manufacturer instructions61. The concentration of RNA was detected using a nano spectrophotometer (Quawell, Q5000 UV–Vis spectrophotometer, San Jose, USA). An equivalent of 1 μg of RNA was transferred to cDNA with the High Capacity cDNA Reverse Transcription Kit® (Applied Biosystems) using random hexamers in a 20 µl reaction volume that was further diluted 1:20 for further downstream analysis.
Quantification of the immune gene using Real-time PCR
Gene expression was assessed by quantitative RT-PCR. Primers for genes that encode inflammation and apoptosis (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) are listed in Table 2, including their sequences and accession numbers in Genbank.
The application of real-time PCR for amplification and relatively quantifying the specified genes in the current study was conducted on an Applied Biosystem Step One (Thermo Fisher Scientific, UK). Real-time PCR was performed using TOPreal qPCR 2 × premix (enzynomics, South Korea) with the following cycling conditions: Initial denaturation at 95 °C for 8 min, followed by 40 cycles of 95 °C for 40 s, 56 °C for 30 s, and 72 °C for 40 s, then the reaction was terminated by a final elongation cycle at 72 °C for 7 min. The expression analysis was done using the 2ΔΔ ct method adopted by Livak and Schmittgen62.
Histomorphometric and immunohistochemical studies
Hematoxylin and eosin staining
Testicular samples were fixed in 10% neutral buffered formalin solution for 24 h. The tissues were then gradually dehydrated with ascending ethanol concentrations, cleaned in xylene, and imbedded in liquid paraffin wax. Using a rotatory microtome, paraffinized blocks were sectioned at a thickness of 5 microns and mounted on either coated glass slides for H & E staining or positive glass slides for immunohistochemical examination63.
Immune-staining of caspase-3 and proliferating cell nuclear antigen (PCNA)
The technique was applied according to Karen Petrosyan et al. (2002)64. Briefly, testicular sections were dewaxed, rehydrated, incubated with 3% hydrogen peroxide at room temperature for 30 min to inhibit endogenous peroxidase activity, and blocked for 15 min with 5% normal goat serum. The sections were then incubated overnight at 4 °C with primary antibodies against either caspase-3 (1:100, 56,046; Santa Cruz Biotechnology, CA, USA) or PCNA (1:500, ab18197; Abcam). Afterwards, the sections were washed, incubated with secondary antibodies, stained with diaminobenzidine, and counterstained with hematoxylin. The mean density of caspase-3 and PCNA expressions was evaluated and expressed as percent using the image analyzer program (version 1.36, NIH, USA).
Histomorphometric analyses
Using the light microscope (40 × magnification), morphometric analyses were performed on randomly selected five stained slides for each group (5 fields/slide). The mean percentage of normal seminiferous tubules of the testes65,66 was determined using the image analyzer program (version 1.36, NIH, USA). Germinal epithelium maturity was graded according to a modified Johnsen’s scoring method67,68. The score, ranging from 1 to 10, was calculated for each animal based on the stage of spermatogenesis. The image analyzer program was used to calculate the mean density of caspase-3 and PCNA immune expression in all groups.
Statistical analysis
The normality of quantitative parameters (apoptotic and inflammatory gene mRNA expression) was visually examined using normal probability plots and the Kolmogorov–Smirnov test. All data are presented as mean standard deviation of the mean (SEM). Duncan's multiple comparison test was used to perform post hoc multiple pairwise comparisons. The effect of TiO2 NPs/chitosan on oxidative stress-antioxidant parameters, expression of apoptotic proteins, and changes in serum testosterone was studied using a mixed model one-way analysis of variance. SAS® was used for statistical analysis (version 9.2, SAS Institute, Cary, NC). For all analyses, the values were considered statistically significant when p < 0.05.
Ethics approval and consent to participate
All used protocols were approved by the Committee on the Ethics of Animal Experiments of the Faculty of Veterinary Medicine, Mansoura University Code No. R/51. All methods were carried out in accordance with relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
09 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-26997-z
References
Alarifi, S. et al. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int. J. Nanomed. 8, 3937–3943 (2013).
Baranowska-Wójcik, E., Szwajgier, D., Oleszczuk, P. & Winiarska-Mieczan, A. Effects of titanium dioxide nanoparticles exposure on human health—a review. Biol. Trace Elem. Res. 193, 118–129 (2020).
Gao, G. et al. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J. Hazard. Mater. 258–259, 133–143 (2013).
Komatsu, T. et al. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol In Vitro 22, 1825–1831 (2008).
Gurr, J.-R., Wang, A. S. S., Chen, C.-H. & Jan, K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213, 66–73 (2005).
De Jong, W. H. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29, 1912–1919 (2008).
Halawa, A. et al. Chitosan attenuated the neurotoxicity-induced titanium dioxide nanoparticles in brain of adult rats. Environ. Toxicol. 37, 612–626 (2022).
Geraets, L. et al. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 11, 30 (2014).
Meena, R. & Kajal, K. Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male wistar rat. Appl. Biochem. Biotechnol. 175, 825–840 (2015).
Al-Baqami, N. M. & Hamza, R. Z. Synergistic antioxidant capacities of vanillin and chitosan nanoparticles against reactive oxygen species, hepatotoxicity, and genotoxicity induced by aging in male Wistar rats. Hum. Exp. Toxicol. 40, 183–202 (2021).
Moura, D., Mano, J. F., Paiva, M. C. & Alves, N. M. Chitosan nanocomposites based on distinct inorganic fillers for biomedical applications. Sci. Technol. Adv. Mater. 17, 626–643 (2016).
Marianti, A., Isnaeni, W., Setiati, N. & Sumadi, S. Effects of chitosan on sperm quality of lead acetate-induced rats. J. Phys. Conf. Series 1567(3), 032061 (2020).
Khorsandi, L., Orazizadeh, M., Moradi-Gharibvand, N., Hemadi, M. & Mansouri, E. Beneficial effects of quercetin on titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environ. Sci. Pollut. Res. Int. 24, 5595–5606 (2017).
Song, G. et al. Toxic effects of anatase titanium dioxide nanoparticles on spermatogenesis and testicles in male mice. Pol. J. Environ. Stud. 26, 2739–2745 (2017).
Jia, F., Sun, Z., Yan, X., Zhou, B. & Wang, J. Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch. Toxicol. 88, 781–788 (2014).
Hussein, M. M. A. et al. Amelioration of titanium dioxide nanoparticle reprotoxicity by the antioxidants morin and rutin. Environ. Sci. Pollut. Res. Int. 26, 29074–29084 (2019).
Karimi, S., Khorsandi, L. & Nejaddehbashi, F. Protective effects of Curcumin on testicular toxicity induced by titanium dioxide nanoparticles in mice. JBRA Assist. Reprod. 23, 344–351 (2019).
Smith, L. B. & Walker, W. H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 30, 2–13 (2014).
Arslan, N. P., Keles, O. N. & Gonul-Baltaci, N. Effect of titanium dioxide and silver nanoparticles on mitochondrial dynamics in mouse testis tissue. Biol. Trace Elem. Res. 200, 1650–1658 (2022).
Ajdary, M. et al. Potential toxicity of nanoparticles on the reproductive system animal models: A review. J. Reprod. Immunol. 148, 103384 (2021).
Yousef, M. I., Abd, H. H., Helmy, Y. M. & Kamel, M.A.-N. Synergistic effect of curcumin and chitosan nanoparticles on nano-hydroxyapatite-induced reproductive toxicity in rats. Environ. Sci. Pollut. Res. Int. 28, 9362–9376 (2021).
El-Megharbel, S. M., Al-Salmi, F. A., Al-Harthi, S., Alsolami, K. & Hamza, R. Z. Chitosan/selenium nanoparticles attenuate diclofenac sodium-induced testicular toxicity in male rats. Curr. Comput.-Aided Drug Des. 11, 1477 (2021).
Chen, Z. et al. Tissue-specific oxidative stress and element distribution after oral exposure to titanium dioxide nanoparticles in rats. Nanoscale 12, 20033–20046 (2020).
Aitken, R. J. & Roman, S. D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 1, 15–24 (2008).
Mancuso, F. et al. Effects of titanium dioxide nanoparticles on porcine prepubertal sertoli cells: An “in vitro” study. Front. Endocrinol. 12, 751915 (2021).
Ni, D.-Q. et al. Titanium dioxide nanoparticles perturb the blood-testis barrier via disruption of actin-based cell adhesive function. Aging 13, 25440–25452 (2021).
Hong, F. et al. TiO2 nanoparticle exposure decreases spermatogenesis via biochemical dysfunctions in the testis of male mice. J. Agric. Food Chem. 63, 7084–7092 (2015).
Altındağ, F. & Özdek, U. Synergistic effects of sinapic acid and ellagic acid ameliorate streptozotocin-induced diabetic nephropathy by inhibiting apoptosis, DNA damage, and structural deterioration in rats. Hum. Exp. Toxicol. 40, S290–S299 (2021).
Ilyas, S. Influence of Chitosan from shrimp skin to quality and quantity of sperm of albino rats after administration of lead. Andrology https://doi.org/10.4172/2167-0250.1000114 (2014).
Franco, R. & Cidlowski, J. A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 16, 1303–1314 (2009).
Franco, R., Schoneveld, O. J., Pappa, A. & Panayiotidis, M. I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 113, 234–258 (2007).
Ismy, J., Sugandi, S., Rachmadi, D., Hardjowijoto, S. & Mustafa, A. The effect of exogenous superoxide dismutase (SOD) on caspase-3 activation and apoptosis induction in pc-3 prostate cancer cells. Res. Rep. Urol. 12, 503–508 (2020).
Kahl, R., Kampkötter, A., Wätjen, W. & Chovolou, Y. Antioxidant enzymes and apoptosis. Drug Metab. Rev. 36, 747–762 (2004).
Rezvani, H. R. et al. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J. Biol. Chem. 281, 17999–18007 (2006).
Su, L.-J. et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 5080843 (2019).
Chau, B. N., Chen, T.-T., Wan, Y. Y., DeGregori, J. & Wang, J. Y. J. Tumor necrosis factor alpha-induced apoptosis requires p73 and c-ABL activation downstream of RB degradation. Mol. Cell. Biol. 24, 4438–4447 (2004).
Ye, L. et al. Toxic effects of TiO2 nanoparticles in primary cultured rat sertoli cells are mediated via a dysregulated Ca2+ /PKC/p38 MAPK/NF-κB cascade. J. Biomed. Mater. Res. A 105, 1374–1382 (2017).
Loveland, K. L. et al. Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front. Endocrinol. 8, 307 (2017).
Zhao, X. et al. Mechanisms of nanosized titanium dioxide-induced testicular oxidative stress and apoptosis in male mice. Part. Fibre Toxicol. 11, 47 (2014).
Hardwick, J. M. & Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a008722 (2013).
Diaz-Ganete, A. et al. Ghrelin’s effects on proinflammatory cytokine mediated apoptosis and their impact on β-cell functionality. Int. J. Endocrinol. 2015, 235727 (2015).
Mohamed, N. R., Badr, T. M. & Elnagar, M. R. (2021) Efficiency of curcumin and chitosan nanoparticles against toxicity of potassium dichromate in male mice. Int. J. Pharm. Pharm. Sci. https://doi.org/10.22159/ijpps.2021v13i2.40224
Sudjarwo, S., Anwar, C., Wardani, G., Eraiko, K. & Koerniasari,. Antioxidant and anti-caspase 3 effect of chitosan-Pinus merkusii extract nanoparticle against lead acetate-induced testicular toxicity in rat. Asian Pacific J. Reprod. 8, 13 (2019).
Park, J. M. et al. Modification of PCNA by ISG15 plays a crucial role in termination of error-prone translesion DNA synthesis. Mol. Cell 54, 626–638 (2014).
Angelopoulou, R. et al. Evaluation of immunohistochemical markers of germ cells’ proliferation in the developing rat testis: A comparative study. Tissue Cell 40, 43–50 (2008).
Steger, K., Aleithe, I., Behre, H. & Bergmann, M. The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: An immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol. Hum. Reprod. 4, 227–233 (1998).
Reda, S., Hashem, H., Elnegris, H. & Elshal, L. Role of bone marrow derived mesenchymal stem cells in protection of spermatogenic and Sertoli cells against histological alterations induced by torsion/detorsion in rats. J. Med. Histol. 1, 154–169 (2017).
Elmallah, M. I. Y., Elkhadragy, M. F., Al-Olayan, E. M. & Abdel Moneim, A. E. Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int. J. Mol. Sci. 18, 957 (2017).
Russell, L. D., Chiarini-Garcia, H., Korsmeyer, S. J. & Knudson, C. M. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol. Reprod. 66, 950–958 (2002).
Braydich-Stolle, L., Hussain, S., Schlager, J. J. & Hofmann, M.-C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 88, 412–419 (2005).
Gromadzka-Ostrowska, J. et al. Silver nanoparticles effects on epididymal sperm in rats. Toxicol. Lett. 214, 251–258 (2012).
Ahotupa, M. & Huhtaniemi, I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally cryptorchid rat testis. Biol. Reprod. 46, 1114–1118 (1992).
Xing, Y. et al. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials 10, 1365 (2020).
Wang, Z.-F. et al. Therapeutic effect of chitosan on CCl4-induced hepatic fibrosis in rats. Mol. Med. Rep. 18, 3211–3218 (2018).
Azim, S. A. A., Darwish, H. A., Rizk, M. Z., Ali, S. A. & Kadry, M. O. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: Possible role of some antioxidants. Exp. Toxicol. Pathol. 67, 305–314 (2015).
Bearden, H. J. & Fuquay, J. W. Applied Animal Reproduction Vol. 1, 157–165 (Reston Pub. Co, 1980).
Draper, H. H. & Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Meth. Enzymol. 186, 421–431 (1990).
Beutler, E., Duron, O. & Kelly, B. M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888 (1963).
Habig, W. H., Pabst, M. J. & Jakoby, W. B. Glutathione S-transferase AA from rat liver. Arch. Biochem. Biophys. 175, 710–716 (1976).
Nishikimi, M., Appaji, N. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854 (1972).
Samy, A. et al. The potential protective and therapeutic effects of platelet-rich plasma on ischemia/reperfusion injury following experimental torsion/detorsion of testis in the Albino rat model. Life Sci. 256, 117982 (2020).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Bancroft, J. D. & Floyd, A. D. Light microscopy. In Bancroft’s Theory and Practice of Histological Techniques 37–68 (Elsevier, 2013). https://doi.org/10.1016/B978-0-7020-4226-3.00003-2.
Petrosyan, K., Tamayo, R. & Joseph, D. Sensitivity of a novel biotin-free detection reagent (Powervision+TM ) for immunohistochemistry. J. Histotechnol. 25, 247–250 (2002).
Oatley, J. M. et al. Changes in spermatogenesis and endocrine function in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J. Anim. Sci. 83, 604–612 (2005).
Orazizadeh, M., Khorsandi, L. S. & Hashemitabar, M. Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia 42, 247–253 (2010).
Johnsen, S. G. Testicular biopsy score count—a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1, 2–25 (1970).
Ozmerdiven, G. et al. The protective effect of L-arginine, tadalafil, and their combination in rat testes after ischemia and reperfusion injury. Can. Urol. Assoc. J. 11, E19–E25 (2017).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.H.: Conceptualization; methodology; software; data curation; writing; editing; supervision. G.E.: Biochemical analysis; data analysis; writing. S.R.; S.L.: Methodology; writing; data curation; analysis histomorphometric and immunohistochemical analyses. M.E.: Conceptualization; methodology; data analysis; writing. N.S.: Conceptualization; writing. E.E.; A.F.: Methodology; nanomaterial; characterization; writing. M.S; S.A; N.A; N.K.S. Funding Acquisition. M.E: designed and supervised the study, contributed to data interpretation and manuscript writing and revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Affiliations. Full information regarding the corrections made can be found in the correction notice for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halawa, A.A., Elshopakey, G.E., Elmetwally, M.A. et al. Impact of chitosan administration on titanium dioxide nanoparticles induced testicular dysfunction. Sci Rep 12, 19667 (2022). https://doi.org/10.1038/s41598-022-22044-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22044-z
This article is cited by
-
Fructose improves titanium dioxide nanoparticles induced alterations in developmental competence of mouse oocytes
BMC Veterinary Research (2024)
-
The renoprotective activity of amikacin–gamma-amino butyric acid–chitosan nanoparticles: a comparative study
Inflammopharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.