Abstract
Gangliogliomas (GGs) are low-grade brain tumours that cause intractable focal epilepsy in children and adults. In GG, as in epileptogenic focal malformations (i.e., tuberous sclerosis complex, TSC), there is evidence of sustained neuroinflammation with involvement of the pro-inflammatory cytokine IL-1β. On the other hand, anti-inflammatory mediators are less studied but bear relevance for understanding seizure mechanisms. Therefore, we investigated the effect of the key anti-inflammatory cytokine IL-10 on GABAergic neurotransmission in GG. We assessed the IL-10 dependent signaling by transcriptomic analysis, immunohistochemistry and performed voltage-clamp recordings on Xenopus oocytes microtransplanted with cell membranes from brain specimens, to overcome the limited availability of acute GG slices. We report that IL-10-related mRNAs were up-regulated in GG and slightly in TSC. Moreover, we found IL-10 receptors are expressed by neurons and astroglia. Furthermore, GABA currents were potentiated significantly by IL-10 in GG. This effect was time and dose-dependent and inhibited by blockade of IL-10 signaling. Notably, in the same tissue, IL-1β reduced GABA current amplitude and prevented the IL-10 effect. These results suggest that in epileptogenic tissue, pro-inflammatory mechanisms of hyperexcitability prevail over key anti-inflammatory pathways enhancing GABAergic inhibition. Hence, boosting the effects of specific anti-inflammatory molecules could resolve inflammation and reduce intractable seizures.
Similar content being viewed by others
Introduction
Gangliogliomas (GGs) are the most frequent tumor type among developmental low-grade brain tumors which are well-recognized causes of intractable focal epilepsy in children and young adults1,2. Accordingly, epileptic seizures are reported in 80–100% of patients with GG compared to 30% in malignant gliomas3. However, the pathophysiological mechanisms of GG epileptogenicity are still poorly understood4,5.
Due to their strong association with epileptic seizures, it was suggested that GGs are endowed of intrinsically altered synaptic functions. This clinical feature aligns with recent findings that the oncogenic BRAF somatic mutation in GG elicits hyperexcitability6 that is mediated by RE1-silencing transcription factor, a master regulator of ion channels and neurotransmitter receptors in epilepsy3, and by the activation of the epileptogenic Akt/mTOR signaling7,8.
One factor likely contributing to GG epileptogenicity relates to neuroinflammation that is described in these lesions4,9, since this phenomenon is involved in both epileptogenesis and ictogenesis10.
Indeed, the expression and receptor signaling of various cytokines undergo changes in epileptic foci, and cytokine levels are often modified in serum and cerebrospinal fluid of patients with epilepsy11. Some of these molecules play a role in seizure generation in animal models by modifying the activity of voltage-gated or receptor-coupled ion channels12,13, and by inducing transcriptional changes of genes involved in synaptic transmission and epileptogenesis10,13. In particular, the prototypical inflammatory cytokine interleukin-1β (IL-1β) plays a pivotal role in ictogenesis and epileptogenesis both in experimental models of epilepsy14,15,16 and patients.
Our investigation stemmed from the observation that the developmental brain tumours, such as GG and TSC cortical tubers represent common causes of drug-resistant focal epilepsy with early seizure onset5. In addition, recent advances highlight the involvement of different, but also converging, epileptogenic mechanisms including the activation of mTOR pathway as well as a sustained inflammatory response in both these lesions with the involvement of the pro-inflammatory cytokine IL-1β17.
The anti-inflammatory cytokine interleukin-10 (IL-10)18,19 has attracted attention in epilepsy as master regulator of glial cell inflammatory phenotypes20. Moreover, IL-10 was shown to reduce IL-1β production and inflammasome activation in experimental epilepsy21 and attenuated behavioral changes induced by chronic administration of IL-1β in rats22. However, scarce information is available on the effects of IL-10 on synaptic transmission and whether IL-10 modulates the neuronal activity as reported for IL-1β23 Cytokines and chemokines may affect Ca2+ permeability of NMDA and AMPA receptors13 and regulate GABAA receptors (GABAARs) trafficking24. Interestingly, while IL-1β decreased the amplitude of GABA-evoked currents23, the chemokine fractalkine (CX3CL1) reduced the GABA current desensitization in temporal lobe epilepsy (TLE), thus resulting in opposite functional effects on GABA neurotransmission25. This evidence suggests that the net effect of neuroinflammation on neuronal network excitability likely depends on the balance between the action of individual cytokines/chemokines and how their effects are compensated for by anti-inflammatory mechanisms13,26. Here, we studied the expression of IL-10 and IL-1β related genes and proteins by transcriptomic analysis and immunohistochemistry in GG as compared with TSC-cortical tubers, highly epileptogenic focal malformations. We performed electrophysiology experiments to study IL-10 and IL-1β effects on GABAergic neurotransmission in order to shed light on the effects of anti-inflammatory and pro-inflammatory stimuli on neurotransmission in epileptogenic lesions.
Results
Differential expression analysis of IL-1β and IL-10 pathway related genes
The IL-10R complex includes the IL-10 binding subunit IL-10Rα and the accessory subunit IL-10Rβ responsible for recruitment of downstream signaling proteins27. IL-10 binding to its receptor leads to the activation of the proximal kinases JAK1-JAK2-TYK2 and subsequently of phosphokinases and the STAT3 system18,27. Therefore, we first carried out a differential gene expression analysis of mRNAs encoding several proteins involved in the IL-10 downstream signaling pathway (Fig. 1) in GG and TSC patients who underwent surgery for drug-resistant epilepsy and compared with control cortex cases (Supplementary Information).
Expression of genes of interest in controls, GG and TSC. RNAseq data indicate significant up-regulation (adjusted p value = 0.05) of IL-10Rα, IL-10Rβ, IL-1Ra, IL-1β and STAT3 in both GG and TSC. In addition, there is a significant upregulation of IL-10 and JAK1 in GG. IL-10 downstream signaling protein such as TIK1, phosphokinases (PIK3CA, PIK3CB, PIK3CD) and IL1-R1 did not show significant changes in either GG or TSC vs controls. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. A linear model was fit for each gene and moderated t-statistic was calculated after applying an empirical Bayes smoothing to the standard errors. Those genes with a Benjamini–Hochberg adjusted p value < 0.05 were considered significant. Differential expression analysis compared 21 TSC patients and 15 matched control cortices; 37 GG patients and 15 matched controls cortices.
We found that IL-10 transcript was significantly upregulated only in GG patients (log2 fold-change, FC = 1.019) (Fig. 1) whilst its receptors (IL-10Rα and IL-10Rβ) and STAT3 were upregulated in both GG (IL-10Rα log2FC = 1.775; IL-10Rβ log2FC = 0.956; STAT3 log2FC = 0.622) and TSC (IL-10Rα log2FC = 1.212; IL-10Rβ log2FC = 0.534; STAT3 log2FC = 0.527) (Fig. 1). As for IL-10 transcript only JAK1 showed significant overexpression in GG (log2FC = 0.303) (Fig. 1) whilst TYK2 and the phosphokinases PIK3CA, PIK3CB, PIK3CD were not differentially expressed in either TSC or GG (Fig. 1).
The IL-1β and IL-1Ra (IL-1 receptor antagonist) were significantly upregulated in both TSC (IL-1β log2FC = 3.846; IL-1Ra log2FC of 1.872) and GG (IL-1β log2FC = 3.832; IL-1Ra log2FC = 2.097) (Fig. 1). Noteworthy, the ratio between IL-1β/IL-1Ra was shifted towards the pro-epileptogenic IL-1β (2.05- and 1.83-fold in TSC and GG, respectively) suggesting that the IL-1β signaling was not efficiently controlled by the required ~ 100-fold excess of IL-1Ra28. IL-1R1 transcript showed no differential expression in epileptogenic lesions versus control tissue (Fig. 1).
Cellular expression of IL-10Rα in GG and TSC
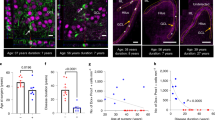
In human control cortex, throughout all cortical layers and white matter, IL-10Rα immunoreactivity was not detectable in neurons or glial cells (Fig. 2A–C). In GG, IL-10Rα immunoreactivity was observed in dysplastic neurons and tumor astrocytes (Fig. 2D, E). Double-labelling showed IL-10Rα expression in neuronal cells (NeuN-positive) and in GFAP-positive astrocytes. In GG, IL-10Rα was also detected in pJAK-positive cells. In TSC, IL-10Rα immunoreactivity was observed in dysmorphic neurons as well as in astrocytes and in scattered giant cells (Fig. 2F–H). Double-labelling showed IL-10Rα expression in neuronal cells (NeuN- and MAP2-positive) as well as in GFAP-positive astrocytes and in dysmorphic neurons positive for pS6, a marker of mTOR activation. Semiquantitative analysis of IL-10Ra immunoreactivity is shown in Supplementary information.
Cellular expression pattern of IL-10 receptor in GG and TSC. Representative photomicrographs of immunocytochemical staining for IL-10Rα. Sections are counterstained with haematoxylin. Panels (A–C): IL-10Rα immunoreactivity (IR) in control brain. No detectable neuronal or glial labelling is observed in normal cortex (A–B; arrows indicate neurons) and white matter (C). Panels (D–E): IL-10Rα in gangliogliomas (GG). IL-10Rα IR was observed in dysplastic neurons (arrows, D and insert-a in E); insert in D shows expression of IL-10Rα (green) in a NeuN positive (red) dysplastic neuron; insert-b in E shows co-localization of IL-10Rα (blue) with pJAK (red). IL-10Rα IR was observed in scattered astroglial cells (arrowheads in E); insert-c in E shows co-localization of IL-10Rα (green) with GFAP (red). Panels (F–H): IL-10Rα in tuberous sclerosis complex (TSC). IL-10Rα IR was observed in dysmorphic neurons (arrows and inserts in F and H); IR was also observed in astrocytes (arrowheads; insert in H-a) and few giant cells (asterisks) within the dysplastic area. Insert in (F) shows expression of IL-10Rα (green) in a NeuN positive (red) dysmorphic neuron. Insert in (G) shows co-localization of IL-10Rα (green) with MAP2 (red). Insert (a) in (H) shows co-localization of IL-10Rα (green) with GFAP (red). Insert (b) in (H) shows co-localization of IL-10Rα (blue) with pS6 (red) in a dysmorphic neuron. Scale bar: (A, D): 100 µm; (F, G): 80 µm; (B, C, E): 40 µm; (H): 30 µm. Details on the cohort used are reported in Supplementary Information.
IL-10 effect on GABAA mediated currents
We determined whether the up-regulation of IL-10 and related signaling was associated with an effect of IL-10 on GABAergic transmission.
First, we used oocytes microinjected with human cDNAs encoding for α1β2γ2 GABAARs (the most common receptor isoform in CNS29) or α4β2γ2 (expressing α4, one of the most relevant subunit mediating tonic inhibition29) to test whether IL-10 affects GABA currents (IGABA) by direct interaction with GABAAR.
IL-10 (100–200 ng/mL) co-applied with GABA 50 μM did not affect IGABA (not shown). Similarly, IL-10 was ineffective on IGABA when oocytes expressing α1β2γ2 were preincubated with the cytokine (100–200 ng/ml) for 3 h (50 μM GABA applied for 4 s; IGABA, 688 ± 431 nA before IL-10; 626.7 ± 379 nA, after 200 ng/ml IL-10; n = 8, Fig. 3). As previously shown23, these evoked currents were completely blocked by bicuculline (100 μM, Fig. 3). Similar results were obtained with oocytes expressing the α4β2γ2 after incubation with IL-10 for 3 h (50 μM GABA applied for 4 s; IGABA 512 ± 270 nA before IL-10; 474 ± 203 nA, after 200 ng/ml IL-10; n = 6). Altogether these results show that there is not a direct interaction with α1 nor with α4 containing GABAA receptors or an activation of endogenous signaling pathways.
IL-10 effect on GABA current amplitude in oocytes injected with human α1β2γ2 cDNA. The bar-graph represents the mean and ± s.e.m. of the IGABA amplitudes evoked from oocytes intranuclearly injected with α1β2γ2 cDNAs before (black) and after (red) the incubation with IL-10 (200 ng/mL, 3 h; n = 8; p > 0.05 by paired t-test).The IGABA amplitudes recorded after the IL-10 incubation were normalized to the response obtained before exposure to IL-10 for each cell (range of current amplitudes: from 247.5 to 1119.0 nA), then averaged and expressed as a percent variation. Traces depict representative currents measured after 4 s application of GABA (white bar, 50 μM) in oocytes injected with α1β2γ2 cDNAs before (black trace) and after (red trace) IL-10 incubation (for 3 h). Grey bar on the right trace represents the block by 100 μM bicuculline (representative of 4 experiments).
Next, we incubated oocytes microinjected with GG tissue (# 8–12; Table 1) with IL-10 (100 ng/ml) for 10 min to 4 h (Fig. 4). We observed a time-dependent increase of IGABA amplitude induced by IL-10. In particular, no changes in IGABA were found up to 40 min incubation, while IGABA amplitude was progressively increased thereafter (+ 8.5 ± 5.4% IGABA amplitude at 120 min; n = 6). The peak increase was attained after 3 h (+ 27.4% ± 3.1% IGABA amplitude; n = 12; p < 0.001) which was maintained up to 4 h incubation (Fig. 4). On the contrary, in the subsequent experiments, we chose to incubate GG microinjected oocytes with IL-10 (100 ng/ml) for 3 h showing an average IGABA increase of + 32.3 ± 2.2% (IGABA = 30.35 ± 2.0 nA before IL-10 and 39.75 ± 2.8 nA after IL-10 incubation, n = 80; p < 0.001; # 8–12 in Table 1; Fig. 5A, B). The IL-10 effect elapsed after 1–3 h wash-out, and it was not related to a change of GABA current reversal potential (EGABA= − 22.9 ± 0.7 before IL-10 and − 22.4 ± 1.6 after IL-10, n = 30; # 8–12 in Table 1) nor to a change of current decay (T0.5 = 13.08 ± 1.7 s before vs. 14.64 ± 0.7 s after IL-10 incubation; p > 0.05, Wilcoxon signed rank test). IL-10 (50 ng/ml) increased IGABA current to a lower extent (+ 19.1 ± 5.0%; n = 6; p < 0.05; # 8–9 in Table 1) while 200 ng/ml IL-10 increased the IGABA current similarly to 100 ng/ml (+ 29.3 ± 4.2%, n = 6; p < 0.05; # 8–9 in Table 1). Altogether, our results show that IL-10 effect in GG was time and dose-dependent.
Time-course effect of IL-10 on GABA current amplitude in oocytes microinjected with GG tissue. GABA alone was applied at 250 μM at the beginning of each experiment (time zero) and at various time points after treatment with 100 ng/ml IL-10. Data (mean ± s.e.m.; n = 6–12 oocytes/time point; patients #8–12 in Table 1) represent the percentage increase of the peak amplitude induced by IL-10. Data were normalized to the mean current amplitude recorded at time zero (23.7 ± 8.6 nA, n = 12). Inset: Traces depict representative GABA currents at the indicated times. * = p < 0.05 by Wilcoxon signed rank test; ** = p < 0.01 by paired t-test.
Characterization and blockade of IL-10 effect on GABA current amplitude. (A) Bar-graph of IL-10 effect on IGABA current amplitude in oocytes microtransplanted with control tissue (n = 17), TSC (n = 24) and GG (n = 80) tissues. Data are expressed as mean ± s.e.m. Inset: IGABA amplitude is expressed as percent increase above baseline (before IL-10 incubation, ranges of current amplitudes: control tissue, from 3.8 to 53.7 nA; TSC, from 7.7 to 73.7 nA; GG, from 5.5 to 84.0 nA). ** = p < 0.01 by paired t-test. (B) Representative superimposed current traces (GABA 250 μM, white bars) of control-, TSC- and GG-injected oocytes before (black trace) and after (red trace) incubation with IL-10 (100 ng/mL for 3 h). Grey bars represent the block by 100 μM bicuculline (representative of 3 experiments for each tissue) (C) Bar-graph shows the effect of incubation of K252a (2 μM, a broad-spectrum protein kinases inhibitor) or baricitinib (Bar 0.5 μM, a selective JAK1 and JAK2 inhibitor) with IL-10 (100 ng/ml). Black bar-graph represents the mean current value (nA) before incubation with IL-10 alone (red, n = 18) or in combination with the two blockers (blue, n = 8 for each blocker). ** = p < 0.01 by paired t-test (D) Dose–response curves of GABA (1 μM–1 mM) before (black curve) and after (red curve) incubation with IL-10 (100 ng/ml for 3 h) in oocytes microinjected with GG tissues (Patients # 8–10, Table 1). Averaged EC50 were 107.0 ± 9.7 μM, nH = 1.4 ± 0.10, before IL-10 and 67.0 ± 3.79 μM, nH = 1.77 ± 0.16; n = 16; statistics for the dose–response experiments: p < 0.05 by paired t-test.
IGABA amplitude was stable in the transplanted oocytes exposed only to incubation medium (see “Methods”) for 3 h showing a mean variation of -5.25% of the control amplitude (time zero, 24.8 ± 2.5 nA versus time 3 h, 23.5 ± 2.4 nA; n = 49; # 8–12 in Table 1). Moreover, IL-10 (100 ng/ml) did not modify IGABA amplitude in oocytes transplanted with control tissues because the treatment determined a not significant average current increase of + 6.4% (IGABA = 28.0 ± 4.5 nA before IL-10 and 29.8 ± 4.1 nA after IL-10, n = 17; Fig. 5A, B).
Next, we used two drugs blocking the downstream signaling activated by IL-1027. Both K252a, a broad spectrum protein kinases inhibitor30 and baricitinib, a selective JAK1 and JAK2 inhibitor31, pre-incubated for 30 min and co-incubated for 3 h with IL-10, prevented the cytokine effect on IGABA current (Table 2 and Fig. 5C). This evidence supports that the increase of IGABA amplitude induced by IL-10 in GG is mediated by the activation of IL-10 signaling axis.
To investigate if the increase of IGABA amplitude in GG was due to a change of GABA affinity, we carried out dose–response GABA experiments before and after 3 h incubation with IL-10 (100 ng/ml). We found a significant leftward shift of the GABA dose–response curve after exposure to the cytokine (GABA EC50 = 106.0 ± 1.5 μM, nH = 1.5 ± 0.1 before IL-10 and 69.7 ± 5.0 μM, nH = 1.7 ± 0.18 after IL-10; # 8–10, Table 1; n = 16; p < 0.05; Fig. 5D) suggesting that IL-10 induces an increase of GABAAR affinity.
Notably, IL-10 (100 ng/ml) incubated for 3 h with oocytes microinjected with TSC tuberal tissue did not induce a significant increase of IGABA amplitude evoked by 250 μM GABA (4 s applications; 29.7 ± 4.0 nA before IL-10 and 32.4 ± 4.5 nA after IL-10 n = 24; # 1–3,5–7 in Table 1; p = 0.29; Fig. 5A, B). This result is compatible with the limited induction of IL-10 signaling in these specimens as compared to GG (Fig. 1).
IL-1β effect on GABAA mediated currents in GG
Since we previously showed that IL-1β inhibited GABAergic transmission in human TLE23, we studied whether this cytokine affects IGABA amplitude in GG and TSC.
In line with our previous findings23, IL-1β (25 ng/mL) after 2 h incubation (peak time of effect) reduced IGABA amplitude in oocytes injected with GG tissue (GABA 250 μM, 4 s applications; 52.3 ± 7.9 nA before IL-1β and 42.7 ± 7.7 nA after IL-1β; n = 17; # 8–12 in Table 1; p < 0.01). Similar results were obtained in oocytes injected with TSC cortical lesional tissues (tubers) (GABA 250 μM, 4 s applications; 52.4 ± 4.0 nA before IL-1β and 38.8 ± 3.8 nA after IL-1β; n = 22; # 1–7 in Table 1; p < 0.001). IL-1β effect was blocked by 30 min pre-incubation with 10 μM IL-1Ra, as assessed in GG (GABA 250 μM, 4 s applications; 39.6 ± 6.8 nA before IL-1β + IL-1Ra and 41.3 ± 9.0 nA after IL-1β + IL-1Ra; n = 8; # 8–11 in Table 1).
IL-1β prevented IL-10 enhancement of GABAA current
We determined the net effect on GABA-evoked currents when oocytes transplanted with GG were exposed to both IL-1β and IL-10, in order to mimic the neuroinflammatory milieu of GG where both cytokines are induced with fold-increase of IL-1β exceeding that of IL-10 (Fig. 1). We pre-incubated oocytes for 30 min with IL-1β (25 ng/ml) and subsequently with a combination of IL-1β (25 ng/ml)23 and IL-10 (100 ng/ml) for further 3 h. IL-10 effect was suppressed by IL-1β at a concentration within the range measured in epilepsy brain tissue32 (GABA 250 μM, 4 s applications; 37.8 ± 5.8 nA before IL-1β + IL-10 and 31.9 ± 4.3 nA after IL-1β + IL-10; n = 10; # 8–10 in Table 1). Notably, we obtained similar results when the pre-incubation was performed with IL-10 before using the same protocol as above (55.6 ± 10.0 nA before IL-10 + IL-1β and 46.4 ± 9.4 nA after IL-10 + IL-1β; n = 9; # 8–9 in Table 1). IL-1β and IL-10 applied alone in the same set of experiments modified IGABA amplitudes as expected (Fig. 6).
Effect of IL-1β on GABA current in the absence or presence of IL-10. Bar-graphs show the % variation in IGABA amplitude induced by IL-10 (red bar and trace), IL-1β (green bar and trace) or their combination (grey bar and trace) in oocytes injected with GG tissues (Patients # 8–10; Table 1). Data are expressed as a % variation of the mean current amplitude after the incubation with each cytokine singularly or in combination. Mean current variation was + 31.0 ± 2.6% after IL-10 incubation (100 ng/ml, n = 10), − 19.6 ± 3.15% after incubation with IL-1β (25 ng/ml, n = 10) and − 15.6 ± 3.5% after co-incubation with IL-10 + IL-1β (n = 10) as described in the text. ** p < 0.01 by paired t-test.
Discussion
Our main objective was to study the role of the anti-inflammatory cytokine IL-10 in neurotransmission underlying GGs epileptogenicity. First, we report the novel evidence that IL-10 and related signaling molecules are up-regulated in GG, and IL-10Rα is induced in neurons and astrocytes. Similarly to GG, IL-10 receptors were induced in TSC and IL-10Rα was expressed by both dysmorphic neurons and astrocytes. However, IL-10 itself and the JAK1 downstream kinase were upregulated in GG but not in TSC, where only STAT3 was induced, suggesting that the IL-10 signaling was activated to a minor extent in TSC compared to GG. Patients with GG and TSC share common characteristics, such as a high incidence of early onset drug-resistant epilepsy and a neuroinflammatory response which is one hallmark of the neuropathology1,5,33,34,35,36. Notwithstanding these common features, IL-10 and related signaling have a significant impact on GABAAR mediated currents in GG but not in TSC, as assessed in oocytes microinjected with membranes from epileptic patients. Data support that the up-regulation of IL-10-related signaling represents a homeostatic attempt to counteract hyperexcitability in epileptogenic lesions by enhancing GABA-mediated currents. However, this up-regulation was insufficient in TSC supporting that the extent of IL-10 increase and cognate signaling activation determine the functional consequences on neurotransmission in epileptiform lesions. In support, IL-10-mediated GABA current potentiation was absent in control tissue where the cytokine and its receptor were undetectable. This evidence bears relevance for therapeutic interventions aimed at boosting IL-10R activation with stable IL-10 analogs or brain penetrant mimetic drugs37. A potential limitation of this study is the use of post-mortem brain tissue as control for surgical resected specimens from GG and TSC. However, comparison of the transcriptional profile has been performed demonstrating minimal variations between the two tissue types when high quality RNA was used as an input38. Moreover, we previously showed that surgical control tissue shows a pattern of immunoreactivity for inflammatory markers very similar to autoptic tissue, thus indicating antigen preservation in control autopsies25,39. In accordance, the use of post-mortem brain material is routinely used in transcriptome and immunohistochemical studies.
We used the microtransplantation approach since it allows to measure GABA currents that are otherwise difficult to record using human brain slices due to the rarity and tissue damage of surgical specimens. Indeed, to our knowledge there are no studies on ex-vivo brain slices in human GG and this is not surprising considering that GG are rare primary brain tumours with a challenging diagnosis that requires integrated diagnostic genotype–phenotype analysis, thus limiting the availability of representative tissue slices for electrophysiological recordings1,40. In addition, there is only one animal model of GG carrying the BRAFV600E mutation where electrophysiological studies on acute brain slices were performed, although modulation of neuronal activity by cytokines has not been evaluated yet3,8.
One limitation of our approach is that we microtrasplanted a mixture of glial or neuronal membranes25 therefore we cannot distinguish whether the IL-10 effect is mediated by glial or neuronal IL-10 receptors. However, the microtransplantation technique bypasses the biosynthetic machinery of host cell allowing the incorporation of native receptors and associated signaling that maintain their functional properties25,41.
Furthermore, this approach permits to use minute amounts of control tissue from individuals without neurological diseases, which is highly relevant when studying neuroinflammatory mediators.
We found that IL-10 increases GABA current amplitude in GG by activating receptor-related kinase cascade, and this effect was dose-dependent and it becomes significant after 3 h incubation. The involvement of the IL-10-related signaling is supported by (i) the blockade of the cytokine effect using drugs interfering with the downstream kinases, and (ii) the lack of IL-10 effect on GABA current in oocytes injected with exogenous cDNAs encoding human α1β2γ2 or α4β2γ2 GABAARs, thus excluding a direct interaction between IL-10 and GABAARs. In agreement, the recruitment of the same JAK/TYK signaling due to the activation of IL-10 receptor complex (IL-10Rα and IL-10Rβ) can trigger an anti-inflammatory axis that reduces neurodegenerative phenomena18.
Direct application of IL-10 on hippocampal naïve rat slices was reported to induce a decrease of peak amplitude and frequency of mIPSCs recorded from DG neurons42. This evidence is only apparently at variance with our results since we measured the IL-10 enhancing effect of GABA amplitude exclusively in pathological cortical tissues, but not in control tissues, suggesting that GABAA receptor subtypes are altered by the pathology, as previously shown in epilepsy patients and animal models43,44.
Notably, the shift of GABA EC50 induced by IL-10, with no changes in reversal potential or current decay, indicates that GABA current potentiation in GG is a consequence of increased receptor affinity for GABA.
We hypothesize that IL-10 could act on tonic GABAergic inhibition which is characterized by high affinity for GABA and the activation of specific GABAAR subunits29,45. Specifically, it is likely that IL-10 could modulate the function of α4 containing GABAA-Rs by acting on subunit phosphorylation or receptor trafficking mechanisms46. Notably, in line with this hypothesis, here we blocked the IL-10 effect by using a broad spectrum kinase inhibitor. A similar role of phosphorylation was previously described for the GABA current potentiation induced by BDNF or levetiracetam in TLE patients both in oocytes and human slices44,47,48. In addition, we described the block of IL-10 effect with a specific inhibitor of JAK1-2 that, together with the reported lack of effect on cDNAs injected oocytes, further supports that the IL-10 signaling machinery is transplanted in the host cells.
Although our approach25 does not allow to determine whether the IL-10 effect is mediated by neuronal or astrocytic receptors, this aspect needs to be elucitated since enhancement of tonic extrasynaptic GABAAR currents may reduce seizures susceptibility45,49.
Previous studies showed an altered chloride homeostasis in peritumoral tissue of low-grade gliomas resulting in depolarizing GABA actions which may contribute to hyperexcitability induced by tumors50,51. Since we did not find any chloride alteration in GG tissues, the enhanced GABA current induced by IL-10 is likely to result in anti-ictogenic effects.
In oocytes transplanted with human TLE membranes, IL-1β reduced GABAAR-mediated currents through activation of its signaling pathway26. Our data show that IL-1β has a similar effect in GG and TSC and this effect was blocked by the specific receptor antagonist IL-1Ra confirming that it was mediated by the activation of the IL-1β receptor and associated molecular cascade as previously reported23. IL-1β is a key component of the neuroinflammatory milieu in epileptogenic tissue10, and its ability to promote neuronal NMDAR-dependent Ca2+ influx and decrease GABA current amplitude likely mediates its ictogenic properties. We provide novel evidence that IL-1β is induced in large excess compared to IL-1Ra in GG supporting the inefficient control of this proinflammatory signal and its pathological consequences10.
The contribution of cytokines to neuroinflammation in epilepsy is complex since various pro- and anti-inflammatory molecules are secreted, and they are often endowed of opposite effects on synaptic transmission and neuronal excitability13,52,53. In line with this scenario, our functional data show that IL-1β prevents the enhancing effect of IL-10 on GABAergic transmission while retaining its ability to decrease GABA currents, thus supporting the failure of anti-inflammatory cytokines to efficiently control neuroinflammation and the consequent hyperexcitability leading to seizures. This hypothesis is also supported by IL-10 serum levels being comparatively lower, and IFN-γ levels higher, in patients with drug-resistant versus drug-responsive epilepsy10,21,54.
Our results reinforce the link between cytokine-mediated neuroinflammation and altered neurotransmission in drug-resistant human epilepsies. In particular, our data suggest that boosting key anti-inflammatory endogenous molecules may represent a novel therapeutic strategy for controlling drug-resistant seizures as also suggested for children with febrile seizures55. In support, recent evidence shows that also the administration of anakinra56,57, the human recombinant IL-1Ra, by increasing the level of endogenous IL-1Ra provides significant therapeutic benefits in drug-resistant patients affected with febrile infection-related epilepsy syndrome.
Conclusions
This study provides fresh evidence that the anti-inflammatory mediator IL-10 affects GABA currents in epileptogenic human tissue, thus bearing implications for novel strategies to increase inhibitory neurotransmission in drug-resistant epilepsy. Since IL-1β voided the effect of IL-10 on GABA currents, this supports that the resolution mechanisms of the pathogenic neuroinflammatory response may fail in epilepsy, thus allowing the ictogenic effects of the concurrent inflammatory molecules to prevail.
Our data provide therapeutic insights for inhibiting hyperexcitability underlying seizures by boosting endogenous anti-inflammatory homeostatic mechanisms with drugs that mimic key anti-inflammatory molecules.
Methods
Patients
The cases included in this study were obtained from the archives of the Departments of Neuropathology of the Amsterdam UMC (Amsterdam, the Netherlands) and the University Medical Center Utrecht (UMCU, Utrecht, the Netherlands). Cortical brain samples were obtained from patients undergoing surgery for drug-resistant epilepsy and diagnosed with GG or TSC (cortical tubers). All cases were reviewed independently by two neuropathologists, and the diagnosis of GG was confirmed according to the revised WHO classification of tumors of the central nervous system58. All patients with cortical tubers fulfilled the diagnostic criteria for TSC59. The predominant seizure types observed were focal seizures with/without impaired awareness, and all patients were resistant to maximal doses of different anti-seizure medications (ASMs) (Table 1 and Supplementary Information). All the patients included in this study had a post-surgical outcome in Engel’s class I or II. Epilepsy duration was calculated as the interval in years from the age at seizure onset to the age at tissue sampling. After resection, the tissue was immediately snap-frozen in liquid nitrogen and then part of the samples was used to perform the electrophysiology experiments. Control autopsy cases had no known history of epilepsy, a normal cortical structure for the corresponding age and no significant brain pathology. All autopsies were performed within 16–48 h after death with the acquisition of appropriate written consent for brain autopsy and subsequent use for research purposes. As pathologies in young patients are investigated, surgically resected control tissue was not available due to technical and ethical issues. The transcriptional profiles of post-mortem and surgical resected tissues have previously been compared to take into account potential post-mortem effects on RNA expression, thus showing minimal differences if the tissue is of high quality (i.e., extracted, handled and stored as in our study)38. Additional details can be found in Supplementary information. The brain specimens used for electrophysiological and immunohistochemical analyses are identified in the text by patient number (“#”) (Table 1). Due to the limited tissue availability of these rare human specimens, we used the frozen samples at completion for both transcriptomic analysis and electrophysiology, thus preventing additional measurements (e.g. western blot) to be done. For electrophysiological experiments, perituberal tissue was available from two TSC patients only and the amount of tissues was insufficient for recording reliable GABA currents amplitudes. Formalin-Fixed Paraffin-Embedded (FFPE) material was used for diagnostic pathology and immunohistochemistry.
Control cortical tissue was obtained from two females (age 7 yrs, intestinal ischemia; 39 yrs, respiratory failure) and one male (age 31 yrs; respiratory failure). Patients and their controls used for electrophysiological and immunohistochemical analyses (Table 1) are part of a larger cohort of GG (37 patients), TSC (21 patients) and controls (15 patients) whose tissue underwent transcriptomic analysis (Supplementary Information).
Membrane preparation
Tissues were immediately processed upon receipt in the laboratory or stored at − 80 °C until use. Human membranes preparation, injection in Xenopus laevis oocytes were carried out as previously described60,61.
Injection and voltage-clamp recordings
Experiments with microtransplanted oocytes were carried out 24–48 h after cytoplasmic injection60 (patients are reported in Table 1). GABA-evoked currents were recorded with the technique of two-electrode voltage clamp as previously reported60 after the oocytes were placed in a recording chamber (0.1 ml volume) and continuously perfused with oocyte Ringer solution (OR: NaCl 82.5 mM; KCl 2.5 mM; CaCl2 2.5 mM; MgCl2 1 mM; Hepes 5 mM, adjusted to pH 7.4 with NaOH) at room temperature (20–22 °C). These GABA currents were blocked by biculline (100 μM) as previously reported62, thus indicating that we recorded genuine GABAA evoked responses23.
In one set of experiments, we used oocytes expressing human α1β2γ2 GABAARs or α4β2γ2 after intranuclear injection of cDNAs encoding human α1 or α4, β2 and γ2 GABAARs subunits63. cDNAs were kindly provided by Dr. K. Wafford and were used at a ratio of 1:1:1.
Unless otherwise specified, 50 μM GABA (plateau dose–response concentration) was used in the experiments with cDNA injected oocytes and 250 μM GABA (plateau dose–response concentration) with microtransplanted oocytes63. The stability of the evoked currents (IGABA) was ascertained by performing two consecutive GABA applications, separated by a 4 min washout. Only the cells that had a < 5% variation of current amplitude were used to test the effect of IL-10 and IL-1β. In some experiments we applied bicuculline (100 μM, 30 s of incubation), a competitive antagonist of GABAARs, to confirm that we recorded genuine GABA-evoked responses as previously shown23.
When performing dose–response relationships (before and after IL-10 incubation), we used GABA concentrations ranging from 1 μM to 1 mM, as previously reported60. GABA pulses were applied every 4 min to avoid receptor desensitization; to determine the half-maximal effect (EC50) data were fitted to Hill equations using least-square routines, as previously described60.
GABA current reversal potential (EGABA) was calculated by constructing current–voltage (I-V) relationships that were elaborated by a linear regression curve-fitting software (Sigmaplot 12, Systat software inc.). GABA current decay time (T0.5) was measured as the time taken for the current to decay from its peak to half-peak value after applying GABA 250 μM for 60 s64.
Cytokines were diluted at the final concentration (specified for each experiment) in Barth’s modified saline solution (88 mM NaCl; 1 mM KCl; 2.4 mM NaHCO3; 10 mM HEPES; 0.82 mM MgSO4; 0.33 mM Ca(NO3)2; 20.41 mM CaCl). IL-10 was purchased from Immunotools GmbH (Friesoythe, Germany), IL-1β was purchased from Peprotech (London, UK) and recombinant human IL-1Ra from Invitrogen (Waltham, MA, USA). Salts were purchased from Sigma-Aldrich (USA) while GABA and biculline methocloride were purchased from Tocris Bioscience (Bristol, UK) and dissolved in sterile water before dilution to the final concentration in OR.
In some experiments, we used K252a (Sigma; 2 μM), a potent non-specific inhibitor of protein kinases such as PKA, PKC, PKG and Trk receptors, and baricitinib (Selleckchem; 0.5 μM), a selective JAK1 and JAK2 inhibitor. Oocytes were incubated for 30 min with the inhibitor alone, followed by co-incubation with the cytokine for 3 h.
Immunohistochemistry
Immunohistochemistry (patients are reported in Table 1) was carried out as previously described65. The primary antibody against IL-10 receptor alpha (IL-10Rα, rabbit polyclonal, Genetex, Irvine, CA, USA, 1:150) was incubated at room temperature for 1 h for single labelling. The ribosomal protein S6 was used as marker of the mTOR activity (pS6 Ser235/6, polyclonal rabbit, Cell Signaling; 1:200) to perform double-labelling of IL-10Rα.
The immunofluorescent labelling was performed as previously described66 by incubating NeuN (mouse monoclonal, clone MAB377; Chemicon, Temecula, CA, USA; 1:2.000), microtubule-associated protein (MAP2; mouse clone HM2; Sigma 1:100) and glial fibrillary acidic protein (GFAP; mouse monoclonal, clone GA5, Sigma-Aldrich, St. Louis, MO, USA; 1:4.000) antibody with the primary antibody against IL-10Rα. Sections were analyzed using Leica Confocal Microscope TCS SP8 X DLS (Leica, Son, the Netherlands) at 20 × magnification (bidirectional X, speed 600 Hz, pinhole 1.00 AU).
RNA-Seq library preparation, sequencing and bioinformatics analysis
All library preparation, sequencing and bioinformatic analyses including differential expression analysis were carried out as previously described65 (patients are reported in Supplementary Information). Differential expression analysis compared 21 TSC patients and 15 age-matched control cortices; 37 GG patients and 15 age-matched controls cortices. The relationship between expression level of differentially expressed RNAs and subject’s age was assesssed using Spearman’s rank correlation to. A correlation coefficient of (adjusted p value < 0.05) > 0.7 or < − 0.7 was considered indicative of meaningful relationship between the two variables. As no significant correlation was found between gene expression levels and subject’s age, it was deemed that no correction for age needed to be applied.
Statistics
Before data analysis, normal distribution was assessed with Shapiro–Wilk test to inform about the choice between parametric (Student's t-test) or non-parametric (Wilcoxon signed rank test, Mann–Whitney rank sum test) tests. Statistical analysis of data was performed with Sigmaplot 12 software, and differences between two data sets were considered significant when p < 0.05. The (n) indicates the number of oocytes used in each experiment.
Ethics approval
Human brain tissue was obtained and used in accordance with the Declaration of Helsinki and the Amsterdam UMC Research Code provided by the Medical Ethics Committee. All the samples were used upon acquisition of appropriate written consent for research purposes. The use of Xenopus laevis frogs, the surgical procedures for oocytes extraction and use conformed to the Italian Ministry of Health guidelines (authorization no 427/2020-PR), and were approved by the Local Committee for Animal Health (OPBA, Department of Physiology and Pharmacology, Sapienza University). All the animal procedures followed the recommendations of the ARRIVE guidelines.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GG:
-
Ganglioglioma
- TSC:
-
Tuberous scleroris complex
- IL-1β:
-
Interleukin-1β
- IL-10:
-
Interleukin-10
- GABAA :
-
γ-Aminobutirric acid type A
- BRAF:
-
B-Raf proto-oncogene
- Akt:
-
Ak strain transforming kinase
- mTOR:
-
Mammalian target of rapamycin
- NMDA:
-
N-methyl-D-aspartate
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CX3CL1:
-
Chemokine (C-X3-C motif) ligand 1
- ASMs:
-
Anti-seizure medications
- IL-1Ra:
-
Interleukin-1 receptor antagonist
- pS6:
-
S6 ribosomal protein
- TYK2:
-
Tyrosine kinase 2
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- PKG:
-
Protein kinase G
- CNS:
-
Central nervous system
- IGABA :
-
GABA current
- EGABA :
-
GABA current reversal potential
- I-V:
-
Current–voltage relationship
- JAK1:
-
Janus activated kinase 1
- JAK2:
-
Janus activated kinase 2
- IL-10Rα:
-
Interleukin-10 receptor α
- IL-10Rβ:
-
Interleukin-10 receptor β
- NeuN:
-
Neuronal nuclei antigen
- MAP2:
-
Microtubule-associated protein 2
- GFAP:
-
Glial fibrillary acidic protein
- STAT3:
-
Signal transducer and activator of transcription 3
- mIPSCs:
-
Miniature inhibitory post-synaptic currents
- DG:
-
Dentate gyrus neurons
- EC50 :
-
Half maximal effective concentration
- IFN-γ:
-
Interferon γ
References
Slegers, R. J. & Blumcke, I. Low-grade developmental and epilepsy associated brain tumors: A critical update 2020. Acta Neuropathol. Commun. 8, 27 (2020).
Ertürk Çetin, Ö., İşler, C., Uzan, M. & Özkara, Ç. Epilepsy-related brain tumors. Seizure 44, 93–97 (2017).
Koh, H. Y. et al. BRAF somatic mutation contributes to intrinsic epileptogenicity in pediatric brain tumors. Nat. Med. 24, 1662–1668 (2018).
Aronica, E. et al. Gene expression profile analysis of epilepsy-associated gangliogliomas. Neuroscience 151, 272–292 (2008).
Blumcke, I. et al. Neocortical development and epilepsy: Insights from focal cortical dysplasia and brain tumours. Lancet Neurol. 20, 943–955 (2021).
Goz, R. U., Akgül, G. & LoTurco, J. J. BRAFV600E expression in neural progenitors results in a hyperexcitable phenotype in neocortical pyramidal neurons. J. Neurophysiol. 123, 2449–2464 (2020).
Prabowo, A. S. et al. BRAF V600E mutation is associated with mTOR signaling activation in glioneuronal tumors. Brain Pathol. 24, 52–66 (2014).
Cases-Cunillera, S. et al. Heterogeneity and excitability of BRAF V600E-induced tumors is determined by Akt/mTOR-signaling state and Trp53-loss. Neuro Oncol. https://doi.org/10.1093/neuonc/noab268 (2021).
Prabowo, A. S. et al. Differential expression and clinical significance of three inflammation-related microRNAs in gangliogliomas. J. Neuroinflammation 12, 97 (2015).
Vezzani, A., Balosso, S. & Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 15, 459–472 (2019).
de Vries, E. E. et al. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 63, 177–190 (2016).
Vezzani, A., Aronica, E., Mazarati, A. & Pittman, Q. J. Epilepsy and brain inflammation. Exp. Neurol. 244, 11–21 (2013).
Vezzani, A. & Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82 (2015).
Maroso, M. et al. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8, 304–315 (2011).
Noe, F. M. et al. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol. Dis. 59, 183–193 (2013).
Ambrogini, P. et al. Neurobiological correlates of alpha-tocopherol antiepileptogenic effects and MicroRNA expression modulation in a rat model of kainate-induced seizures. Mol. Neurobiol. 55, 7822–7838 (2018).
Curatolo, P., Specchio, N. & Aronica, E. Advances in the genetics and neuropathology of tuberous sclerosis complex: Edging closer to targeted therapy. Lancet Neurol. 21, 843–856 (2022).
Porro, C., Cianciulli, A. & Panaro, M. A. The regulatory role of IL-10 in neurodegenerative diseases. Biomolecules 10, E1017 (2020).
Saraiva, M. & O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10, 170–181 (2010).
Morin-Brureau, M. et al. Microglial phenotypes in the human epileptic temporal lobe. Brain 141, 3343–3360 (2018).
Sun, Y. et al. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflammation 16, 66 (2019).
Zhang, Y.-P. et al. Interleukin-10 attenuates behavioral, immune and neurotrophin changes induced by chronic central administration of interleukin-1β in rats. NeuroImmunoModulation https://doi.org/10.1159/000521710 (2022).
Roseti, C. et al. GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol. Dis. 82, 311–320 (2015).
Stellwagen, D., Beattie, E. C., Seo, J. Y. & Malenka, R. C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 25, 3219–3228 (2005).
Roseti, C. et al. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia 54, 1834–1844 (2013).
Oto, J. et al. Plasma proinflammatory and anti-inflammatory cytokine and catecholamine concentrations as predictors of neurological outcome in acute stroke patients. J. Anesth. 22, 207–212 (2008).
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Ravizza, T. et al. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol. Dis. 24, 128–143 (2006).
Chuang, S.-H. & Reddy, D. S. Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: Therapeutic insights for epilepsy. J. Pharmacol. Exp. Ther. 364, 180–197 (2018).
Tamura, M., Koyama, R., Ikegaya, Y., Matsuki, N. & Yamada, M. K. K252a, an inhibitor of Trk, disturbs pathfinding of hippocampal mossy fibers. NeuroReport 17, 481–486 (2006).
Roskoski, R. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 111, 784–803 (2016).
Bartfai, T. et al. Interleukin-1 system in CNS stress: Seizures, fever, and neurotrauma. Ann. N. Y. Acad. Sci. 1113, 173–177 (2007).
Wong, M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia 49, 8–21 (2008).
Prabowo, A. S. et al. Differential expression of major histocompatibility complex class I in developmental glioneuronal lesions. J. Neuroinflammation 10, 12 (2013).
Zhang, B., Zou, J., Rensing, N. R., Yang, M. & Wong, M. Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex. Neurobiol. Dis. 80, 70–79 (2015).
Specchio, N. et al. Surgery for drug-resistant tuberous sclerosis complex-associated epilepsy: Who, when, and what. Epileptic Disord. 23, 53–73 (2021).
Chamberlain, T. C. et al. Interleukin-10 and small molecule SHIP1 allosteric regulators trigger anti-inflammatory effects through SHIP1/STAT3 complexes. iScience 23, 101433 (2020).
Mills, J. D. et al. Coding and non-coding transcriptome of mesial temporal lobe epilepsy: Critical role of small non-coding RNAs. Neurobiol. Dis. 134, 104612 (2020).
Ruffolo, G. et al. Functional aspects of early brain development are preserved in tuberous sclerosis complex (TSC) epileptogenic lesions. Neurobiol. Dis. 95, 93–101 (2016).
Blümcke, I. et al. Review: Challenges in the histopathological classification of ganglioglioma and DNT: Microscopic agreement studies and a preliminary genotype-phenotype analysis. Neuropathol. Appl. Neurobiol. 45, 95–107 (2019).
Palma, E. et al. Microtransplantation of membranes from cultured cells to Xenopus oocytes: a method to study neurotransmitter receptors embedded in native lipids. Proc. Natl. Acad. Sci. U.S.A. 100, 2896–2900 (2003).
Suryanarayanan, A. et al. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology 107, 181–188 (2016).
Brooks-Kayal, A. R., Shumate, M. D., Jin, H., Rikhter, T. Y. & Coulter, D. A. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 4, 1166–1172 (1998).
Palma, E. et al. Phosphatase inhibitors remove the run-down of gamma-aminobutyric acid type A receptors in the human epileptic brain. Proc. Natl. Acad. Sci. U.S.A. 101, 10183–10188 (2004).
Farrant, M. & Nusser, Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 6, 215–229 (2005).
Brandon, N. J. et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J. Biol. Chem. 275, 38856–38862 (2000).
Ragozzino, D. et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc. Natl. Acad. Sci. U.S.A. 102, 15219–15223 (2005).
Palma, E. et al. The antiepileptic drug levetiracetam stabilizes the human epileptic GABAA receptors upon repetitive activation. Epilepsia 48, 1842–1849 (2007).
Müller, J. et al. Astrocytic GABA accumulation in experimental temporal lobe epilepsy. Front Neurol 11, 614923 (2020).
Conti, L. et al. Anomalous levels of Cl- transporters cause a decrease of GABAergic inhibition in human peritumoral epileptic cortex. Epilepsia 52, 1635–1644 (2011).
Pallud, J. et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med 6, 244ra89 (2014).
Hulkkonen, J. et al. The balance of inhibitory and excitatory cytokines is differently regulated in vivo and in vitro among therapy resistant epilepsy patients. Epilepsy Res. 59, 199–205 (2004).
Gallentine, W. B. et al. Plasma cytokines associated with febrile status epilepticus in children: A potential biomarker for acute hippocampal injury. Epilepsia 58, 1102–1111 (2017).
Basnyat, P. et al. Chronically reduced IL-10 plasma levels are associated with hippocampal sclerosis in temporal lobe epilepsy patients. BMC Neurol. 20, 241 (2020).
Kim, K. et al. Analysis of plasma multiplex cytokines and increased level of IL-10 and IL-1Ra cytokines in febrile seizures. J. Neuroinflammation 14, 200 (2017).
Kenney-Jung, D. L. et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann. Neurol. 80, 939–945 (2016).
Lai, Y.-C. et al. Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann. Clin. Transl. Neurol. 7, 2467–2474 (2020).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 23, 1231–1251 (2021).
Northrup, H. et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr. Neurol. 123, 50–66 (2021).
Palma, E. et al. Expression of human epileptic temporal lobe neurotransmitter receptors in Xenopus oocytes: An innovative approach to study epilepsy. Proc. Natl. Acad. Sci. U. S. A. 99, 15078–15083 (2002).
Miledi, R., Palma, E. & Eusebi, F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: New procedure for ion channel studies. Methods Mol. Biol. 322, 347–355 (2006).
Scalise, S. et al. Human iPSC modeling of genetic febrile seizure reveals aberrant molecular and physiological features underlying an impaired neuronal activity. Biomedicines 10, 1075 (2022).
Cifelli, P. et al. Dissecting the molecular determinants of gabaa receptors current rundown, a hallmark of refractory human epilepsy. Brain Sci. 11, 441 (2021).
Ruffolo, G. et al. A novel action of lacosamide on GABAA currents sets the ground for a synergic interaction with levetiracetam in treatment of epilepsy. Neurobiol. Dis. 115, 59–68 (2018).
Korotkov, A. et al. Down-regulation of the brain-specific cell-adhesion molecule contactin-3 in tuberous sclerosis complex during the early postnatal period. J. Neurodev. Disord. 14, 8 (2022).
Gruber, V.-E. et al. Impaired myelin production due to an intrinsic failure of oligodendrocytes in mTORpathies. Neuropathol. Appl. Neurobiol. 47, 812–825 (2021).
Acknowledgements
The work was supported by grants from Ateneo Project (Sapienza University)”, grant n° RM11916B84D24429 (EP) and RG12117A8697DCF1 (EP, GR) and AICE-FIRE 2022 (GR). This project has received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 952455; EpiEpiNet (EP, EA). GR was supported by Italian Ministry of Health “Ricerca corrente”. This research was also supported by intramural “DISCAB” GRANT 2022 code 07_DG_2022_05 to PC.
Author information
Authors and Affiliations
Contributions
E.P., E.A., and A.V. contributed to the conception and design of the study; G.R., V.A., A.R., T.Z., J.D.M., P.C., A.G., A.M., J.A., An.M., E.A. and E.P. contributed to the acquisition and analysis of data; G.R., E.P., E.A., A.R., J.D.M., and A.V. contributed to drafting the text or preparing the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruffolo, G., Alfano, V., Romagnolo, A. et al. GABAA receptor function is enhanced by Interleukin-10 in human epileptogenic gangliogliomas and its effect is counteracted by Interleukin-1β. Sci Rep 12, 17956 (2022). https://doi.org/10.1038/s41598-022-22806-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22806-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.