Abstract
Chemotherapy-related thrombocytopenia (CIT) is a significant adverse event during chemotherapy, which can lead to reduced relative dose intensity, increased risk of serious bleeding and additional medical expenditure. Herein, we aimed to develop and validate a predictive nomogram model for prediction of CIT in patients with solid tumor. From Jun 1, 2018 to Sep 9, 2021, a total of 1541 patients who received 5750 cycles of chemotherapy were retrospectively enrolled. Cox regression analysis was performed to identify predictive factors to establish the nomogram model for CIT. The incidence of chemotherapy-induced thrombocytopenia was 21.03% for patient-based and 10.26% for cycles of chemotherapy. The top five solid tumors with CIT are cervix, gastric, bladder, biliary systemic, and ovarian. The incidence of chemotherapy dose delays in any cycle because of CIT was 5.39%. Multivariate analysis showed that tumor site, treatment line, AST, oxaliplatin, and capecitabine were significantly associated with CIT. Moreover, we established a nomogram model for CIT probability prediction, and the model was well calibrated (Hosme-Lemeshow P = 0.230) and the area under the receiver operating characteristic curve was 0.844 (Sensitivity was 0.625, Specificity was 0.901). We developed a predictive model for chemotherapy-induced thrombocytopenia based on readily available and easily assessable clinical characteristics. The predictive model based on clinical and laboratory indices represents a promising tool in the prediction of CIT, which might complement the clinical management of thrombocytopenia.
Similar content being viewed by others
Introduction
Cancer is a major public health problem worldwide. In 2021, 1,898,160 new cancer cases and 608,570 cancer deaths are projected to occur in the world1. Chemotherapy is still one of the main means of treatment for patients with locally advanced or metastatic cancer. Chemotherapy-induced thrombocytopenia (CIT) is defined as a peripheral platelet count less than 100 × 109/L, with or without bleeding in cancer patients receiving myelosuppressive chemotherapy2. Prior studies estimated that more than 30% of patients with a solid tumor experienced thrombocytopenia3,4, of whom over 10% were Grade II and above5.CIT is a significant medical problem during chemotherapy, and it can lead to chemotherapy dose delays, dose reductions, and increased risk of serious bleeding events and additional medical expenditure6,7. In addition, multiple studies suggests that patients who receive lower chemotherapy dose intensity than planned experience worse overall survival (OS), progression-free survival (PFS) and disease-free survival than those receiving standard or dose-dense chemotherapy4,8,9,10.
Until now, the treatment options of CIT are still limited. Patients with platelet transfusion may develop allergic, febrile reactions and acquire infectious diseases11,12. Recombinant human interleukin-11 (rhIL-11) can either directly or indirectly induce cardiotoxicity13,14. The incidence of atrial fibrillation induced by rhIL-11 was about 0.49%15. And there has also been a case report of heart failure caused by rhIL-1116. So, rhIL-11 should be used cautiously when patients have congestive heart failure and atrial arrhythmia. The ecombinant human thrombopoietin (rhTPO) is the only thrombopoietin receptor agonist (TPO–RA) that receives market approval in China for the treatment of CIT. But repeated use of rhTPO can easily cross-react with endogenous TPO to produce anti-drug antibodies18. It seriously hinders the smooth implementation of anti-tumor therapy.
As is known to us all, the incidence and degree of CIT are various in patients with different tumors receiving diverse chemotherapy regimens. However, the data of CIT from clinical trials usually do not reflect the actual proportion of chemotherapy dose delays or dose reductions resulted from thrombocytopenia alone. In addition, patients with severe thrombocytopenia may be withdrawn from the clinical trial, which means that the relationship between CIT and survival cannot be confirmed experimentally. Thus, the clinical trial data is not a perfect substitute for the real- world setting. According to the actual condition and willingness of patients, the Real-World Study (RWS) focused on significant endpoint and conducted long-term follow-up in a large population. The incidence of malignancies is regionally characteristic, but some CIT-related RWS were not regionally generalized3,4,7,19,20, and should always be taken into consideration in geographic differences of CIT. In addition, some studies were aimed at clarifying the relationship between CIT and demographic characteristics and treatments, identifying CIT high-risk population and establishing CIT prediction model to guide clinical treatment in turn. However, the inclusion of risk factors was relatively simple21,22,23.
In the present study, we established a CIT prediction model based on the evaluation of the value of tumor site, treatment regimens, treatment line, TBIL level, AST level, hemoglobin and platelet counts, it will be a viable alternative to clinical diagnosis and therapy.
Methods and materials
Patients and clinical data collection
A retrospective cohort consisting of patients who received chemotherapy for solid tumour treatment, was selected from among all patients who received anti-tumor treatment in the Oncology Department of Jinhua Municipal Central Hospital from June, 2018 to September, 2021. In this study, the primary endpoint was a predictive model for chemotherapy-induced thrombocytopenia.
The inclusion criteria were as following: (1) Histologically or cytologically confirmed solid tumors were enrolled. (2) All patients were followed during all chemotherapy cycles. (3) All enrolled patients had at least one platelet count after the first occurrence of CIT. The exclusion criterions were as following: (1) Exclusion criteria included a history of hematologic malignancy(Excluding lymphoma), confirmed by fluorescence in situ hybridization (FISH) from bone marrow aspirate and biopsy or peripheral blood test in the prior 3 months. (2) A history of a prior symptomatic venous thromboembolic event (VTE) or arterial event. (3) Other reasons for exclusion included any serious concomitant medical condition that could interfere with the conduct of the clinical trial, such as unstable angina, renal failure requiring hemodialysis, or active infection requiring intravenous antibiotics. Tumors with less than 30 patients were classified as “Other tumor site”, and those with 2 or more primary sites were defined as multiple primary cancers(MPC). This study complies with the ethical standards of the Institutional Research Council and the Declaration of Helsinki. The occurrence of CIT(a platelet count < 100,000/ml) during all chemotherapy cycles was considered as the study outcome. Contrary to the traditional definition, we defined the first anticancer treatment at our center as first-line chemotherapy, and so on. To identify the CIT, a platelet count test was performed at the beginning of every chemotherapy cycle. Then, medical records of patients and hospital information system were reviewed.
Study variables and outcome data assays
Medical records of solid tumor patients and hospital information system (the Haitai electronic case system and the Guide Patients Support Care (GPS) information system) were reviewed. The following parameters and measurement methods were used throughout the study.
Variables definitions
Laboratory indicators
The clinically relevant CIT was defined as a platelet (PLT) count < 100,000/ml. The grades of thrombocytopenia were divided into grade 1 to 4. Respectively, 75,000/ml ≤ PLT < 100,000/ml was considered as grade 1, 50,000/ml ≤ PLT < 75,000/ml as grade 2, 25,000/ml ≤ PLT < 50,000/ml as grade 3, and PLT < 25,000/ml as grade 4. The definition of anemia was hemoglobin (HB) concentration < 110 g/L. The definition of leucopenia was white blood cell (WBC) count < 4,000/ml. The C-reactive protein (CRP) level > 8 mg/dL was considered as an increase in CRP. A total bilirubin (TBIL) level < 25 umol/L was considered as a decrease in bilirubin. Albumin (ALB) level < 30 g/L was considered as hypoalbuminemia. An increase in alanine aminotransferase was defined as alanine aminotransferase (ALT) level > 50 U/L and an increase in aspartate aminotransferase (AST) was defined as aspartate aminotransferase level > 35U/L.
Nutrition assessment tools
Body mass index (BMI, classified based on World Health Organization criteria), Patient-Generated Subjective Global Assessment (PG–SGA) score and Nutritional Risk Screening 2002 (NRS2002) score. Severe malnutrition was defined as body mass index (BMI) < 18.5 kg/m2. The final score of NRS2002 ranges from 0 to 7, with a score of ≥ 3 indicating a high nutritional risk. Based on the above assessments, patients are classified as well-nourished (PG–SGA A), moderately or suspected of being malnourished (PG–SGA B), or severely malnourished (PG–SGA C). PGSGA score < 4 was defined as PG–SGA A, 4 ≤ PGSGA score < 8 was defined as PG–SGA B and PGSGA score ≥ 8 defined as PG–SGA C.
Treatment line
The first time a patient came to our center for antineoplastic therapy was defined as first-line therapy, which was different from the traditional definition. It is defined as a line change treatment when the difference between treatment plans is greater than 50%. If the treatment plan was a line change treatment compared with the last treatment, the treatment line this time was defined as the original number of treatment line plus one.
Type of antineoplastic drugs
Typical Alkylating agents(cyclophosphamide, iso-cyclophosphamide, etc.), Platinum compounds(cisplatin, carboplatin, oxaliplatin, etc.), Antimetabolites(fluorouracil, capecitabine, S-1, TAS102, gemcitabine, pemetrexed), Plant alkaloids(taxane, vinorelbine, irinotecan, etoposide, etc.), Cytotoxic antibiotics and related substances(anthracycline, etc.), Hormones(tamoxifen, letrozole, fulvestrant, etc.), Immune Checkpoint Inhibitors (ICIs)(pembrolizumab, nivolumab, duvalizumab, atezolizumab, etc.), Monoclonal antibody(bevacizumab, cetuximab, trastuzumab, TDM1, etc.), Tyrosine Kinase Inhibitor(TKI)(gefitinib, osimertinib, crizotinib, pyrotinib, anlotinib, fruquintinib, etc.).
Statistical analysis
Statistical analyses were performed by R software (version 3.3.2). Continuous variables were categorized or dichotomized for the analyses. Chi-square test and two-tailed test were used for categorical variables and continuous variables respectively. Univariable analysis was performed to identify potential predictors of thrombocytopenia. Factors shown to be significant predictors in univariate analysis (P < 0.05) were brought forward to a multivariate analysis by a backward stepwise procedure.
Nomogram was used to display the clinical prediction model which was constructed based on the results of multivariate analysis for CIT using the package of rms in R software. The predictive accuracy of the final model was quantified using discrimination and calibration measures. The discrimination and calibration of the model were evaluated using the area under the receiver operating characteristic (ROC) curve. A bootstrapping technique was applied using 1000 random data sets (validation set) generated from the original data.
Studies involving animal subjects
Generated Statement: No animal studies are presented in this manuscript.
Studies involving human subjects
Generated Statement: The studies involving human participants were reviewed and approved by Medical Ethics Committee of Affiliated Jinhua Hospital, Zhejiang University School of Medicine (Zhejiang, China). The patients/participants provided their written informed consent to participate in this study.
Inclusion of identifiable human data
Generated Statement: No potentially identifiable human images or data is presented in this study.
Result
Risk factors of CIT based on patients
Initially, we identified 1540 patients with solid tumors receiving chemotherapy during the study period, of which 883 (57.30%) were males and 658 (42.70%) were females. The detailed patients’ demographic and clinical characteristics were summarized in Table 1. Thrombocytopenia occurred in 324 patients, resulting in a frequency of 21.03%. The 5 most common types of cancers are (in the order of frequency) cervix (8, 33.33%), gastric (32, 31.07%), bladder (8, 28.57%), biliary systemic (8, 27.59%), and ovarian (10, 27.03%). Incidences of grade 1, 2, 3, and 4 thrombocytopenia in patients with solid tumors was 50.9%, 32.7%, 12.7%, 3.7%, respectively (Fig. 1). No bleeding was found in the patients.
During the follow-up, CIT only happened once for 189 (58.33%) patients, but 135(41.67%) patients experienced repeated CIT. In the analysis of the number and time of occurrence of CIT, we found that one patient had 16 times of CIT during the anti-tumor treatment, and 9 (2.78%) patients had more than 10 times of CIT (Fig. 2). CIT first appeared when the patient received 5 cycles of chemotherapy. And the PLT decreased to the lowest during the fourth cycle. The lowest count of PLT in 66 (41.8%) patients appeared after the last cycle of chemotherapy. Chemotherapy cycle delay/dose was examined in 81 cancer patients (349 cycles) with complete chemotherapy dose information in the database included myelosuppression, infection, fatigue, severe electrolyte disturbances and liver function injury, among which 45.7% (37/81) were only caused by thrombocytopenia, and grade 3 thrombocytopenia accounted for 43.2%(16/37). In addition, 8 patients had Grade 4 thrombocytopenia, but none had bleeding. RDI in any cycle of the course was 5.39%.
Risk factors of CIT based on chemotherapy cycles
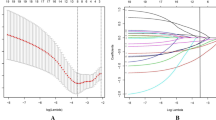
The data were based on 5750 chemotherapy cycles, CIT occurred in 590(10.26%) cycles (Table 2). Patients who received S-1, capecitabine, oxaliplatin, and anthracycline based regimens had a higher incidence of thrombocytopenia (59, 20.34%; 95, 18.13%; 222, 16.78% and 20, 16.53%, respectively) than those who received etoposide, taxanes, gemcitabine, irinotecan and fluorouracil based regimens (15, 7.94%; 79, 7.63%; 37, 7.4%; 98, 7.31% and 102, 6.63%, respectively) (Table 3, Fig. 3). Quite remarkably, TKIs including fruquintinib were significantly related to CIT (P = 0.001, P = 0.002, respectively). CIT, meanwhile, occurred in patients who have been treated with monoclonal antibody and ICIs (233, 9.28% and 46, 8.85%, respectively).
Univariate analysis showed that liver metastasis (P = 0.029), AST (P < 0.001), Tbil (P < 0.001), ALB (P < 0.001), blood cell counts at the last cycle (PLT, P < 0.001; WBC, P < 0.001; HB, P < 0.001), and CRP (P = 0.05) were associated with CIT of patients with solid tumors. The results also showed all chemotherapeutic agents based regimens were closely related to CIT (P < 0.05). According to multivariate Logistic regression analysis, it was noticed that AST, Tbil, blood cell counts at the last cycle, oxaliplatin, alkylating agents, pemetrexed, fluorouracil, capecitabine, anthracycline, TKI excluding fruquintinib, and taxane were significantly associated with CIT (Table 4). Considering that patients may have repeated thrombocytopenia during chemotherapy, the probability of CIT may further increase with the increase of chemotherapy times, and different patients have different bone marrow tolerance to the same chemotherapy regimen, balancing the influence of chemotherapy times and chemotherapy regimen, this study is based on further statistical analysis of patients. Based on the original data, we have resubmitted the appendix.
Nomogram for prediction of CIT
In this study, in order to predict the occurrence of CIT, the independent prognostic factors of tumor site, antineoplastic drugs, blood cell counts at the last cycle (RBC, HB, PLT), CRP, liver metastasis and treatment line were used to establish a nomogram model for CIT probability prediction (Fig. 4). A weighted total score calculated from these variables was used to estimate the occurrence of CIT. In these variables, each factor was assigned a number of risk points, which was obtained by drawing a straight line directly upward to the “points” axis from the corresponding value of the prognostic factor. The process was repeated for each prognostic factor. The points obtained for each covariate were summarized, and “Total Point” axis was located from the sum of the risk points. Finally, a vertical line was drawn directly down to the axis that determined the patient’s probability of CIT. The area under the ROC curve (AUC) value of 0.844 (95% CI: 0.626 to 0.901) indicates that the prediction model has good prediction accuracy (Fig. 5). In addition, the calibration plots for the probability of CIT revealed a good match between the prediction of CIT in the nomogram model and the actual observation (Fig. 6).
Discussion
Patients with solid tumors who received anti-tumor treatment were enrolled in the retrospective cohort. The incidence of CIT in patients with solid tumors was 21.03%, which was 10.26% if based on chemotherapy cycles. The tumor site, treatment line, AST, blood cell counts at the last cycle, oxaliplatin and capecitabine were significantly associated with CIT. In this study, nomogram for prediction of CIT was established on the cohort of more than 1000 patients with solid tumors or lymphomas receiving chemotherapy. The area under the ROC curve (AUC) value of 0.844 indicates that the model has better predictive performance. In addition, the calibration plots for the probability of CIT revealed a good match between the prediction of CIT in the nomogram model and the actual observation.
In our cohort, three common cancers are cervix, gastric and bladder. Unlike Wu's study, the most common cancer was breast cancer (19.5%)4. A study explored the incidence and clinical consequences of chemotherapy-induced thrombocytopenia (CIT) among metastatic colorectal cancer (mCRC) patients, and revealed that 37% of mCRC patients experienced CIT24. These figures for CRC are significantly higher than our results (21.4%). The incidence of thrombocytopenia was ranging from 25% to 27.5%25. This result was slightly less than the incidence of CIT (30.07%) in gastric cancer patients in our study. The incidence of CIT in lung cancer was 12.96%, compared to the treatment caused similar risk of CIT in other clinical studies (ranging from 1 to 23%)26,27,28,29,30.
The occurrence of CIT is closely related to chemotherapy regimens and chemotherapy cycles. The incidence of CIT in platinum based regimens is 13.8%. It was lower to another large observational study of 62,072 chemotherapy regimens, the incidence of platinum-associated CIT in solid tumors was 31%4. Taking a look at three kinds of platinum, our study found that oxaliplatin was significantly associated with CIT (16.8%), which was similar to previous studies25,31,32,33.
The prevalence of CIT after initiation of chemotherapy was 7.4% in patients treated with gemcitabine-based regimens. Previous studies have shown CIT in more than 20% of patients receiving gemcitabine27,34,35. Maarten J. Ten Berg found that the incidence of CIT was 64.4% if patients treated with gemcitabine36. It was significantly higher than our study. Furthermore, multivariate analysis in this study showed that gemcitabine was not associated with CIT. A review of real-world data on 215,508 patients with cancer found that the incidence of gemcitabine-containing regimens in hospitalized patients was only 5.5%20, which is comparable with the present result. In our study, most chemotherapeutic drugs for solid tumor were involved, and all patients were followed during all chemotherapy cycles. Therefore, the result of this study can reflect those who suffered CIT during chemotherapy in eastern China.
In this study, 5-FU-based regimens did not increase the occurrence of CIT, while capecitabine did the opposite. Previous studies have shown that in gastrointestinal tumors, the incidence of 5-FU-related CIT was from 1.49% to 19.0%31,32, which was similar to the results of our study. For capecitabine, however, the incidence of CIT was found to be similar to that of 5-FU37. Coincidentally, the incidence of CIT increased in oxaliplatin-based chemotherapy25,31,33. Our real world data also confirmed that oxaliplatin was positively correlated with CIT. This suggests that for intractable CIT induced by XELOX, We could try replacing it with FOLFOX. Of course, we could suspend oxaliplatin and continue capecitabine, and the next cycle of combination therapy can be carried out after platelet recovery.
For most chemotherapy regimens, CIT first appeared when the patient received 5 cycles of chemotherapy. Jaime et al. found that patients with solid tumor who underwent chemotherapy developed CIT within 3 months19. Moreover, the incidence of CIT went up with the cycles of chemotherapy, which was similar to the results of real-world studies in other regions. In a retrospective study, Bassam et al. found that CIT was positively correlated with the number of chemotherapy cycle. The incidence of CIT was up to 43.8% when the number of chemotherapy cycles ≥ 47.
This study was the first to find that elevated level of AST was associated with CIT. The common drugs which induced acute liver injuries was antineoplastics (15.3%)38. The most important being development of chemotherapy associated liver injuries (CALI)39,40,41,42. The evolution character of CALI was usually accompanied by elevation of ALT, AST, and Tbil. Further research is needed to confirm the intrinsic relationship between them.
At present, only a few studies have established CIT predictive model based on clinical and laboratory variables. In this study, we established a nomogram model for CIT probability prediction in patients with solid tumors (AUC 0.844). In another study, Tanriverdi et al. presented a predictive model based on a single laboratory variable of baseline plasma D-dimer level (an important marker of thrombotic activity) for CIT in patients with sensitivity, specificity, PLR and NLR values of 0.914, 0.897, 3.64 and 0.2422. A total of 14 covariates were prospectively assessed as explanatory variables in a cohort of consecutive patients by Razzaghdoust et al23. The model performance characteristics of the study include sensitivity 75%, specificity 65.4%, positive likelihood ratio 2.16, and negative likelihood ratio 0.38223. The multivariable model exhibited three final predictors for CIT, including body mass index (BMI) < 23 kg/m2 (odds ratio, 2.23; bootstrap P = 0.044). However, BMI did not appear to be associated with CIT in our study.
Based on readily available and easily measurable pretreatment factors, our predictive model may help to distinguish a group of patients at high risk for CIT and those who might benefit from prophylactic administration of thrombopoietic growth factors. However, several limitations should be mentioned. CIT events were identified based on the evidence of either thrombocytopenia during hospitalization or documented medical encounters during bleeding rather than through laboratory results, and it may not reflect the overall incidence of CIT owing to lack of PLT count of out-patients. Most of the patients in this study received gemcitabine on day 8 in outpatient department, from whom we could not obtain the result of blood routine test. That may be the main reason for the relatively low incidence of gemcitabine-related CIT in the present study. And it could be partly explained by the fact that platelet data in this study were collected before the start of the next cycle of chemotherapy from those who had passed the myelosuppression phase. In addition, our model was based on medical records of patients in a single center. Since a single population is not fully representative of the whole population, external validation especially in a community setting will be critical to further demonstrate generality and utility of the model.
In conclusion, we assessed the associations of a panel of clinical variables, available in routine clinical practice, with the occurrence of CIT in the cohort of cancer patients with different solid tumors. There were 11 final variables associated with CIT in the nomogram for prediction of CIT. The prediction model could help to identify patients at risk of CIT and complement the clinical management of thrombocytopenia, but further larger studies are required before clinical application.
Data availability
Generated Statement: The raw data in this study were all from the electronic medical record system of hospitalization. All the original data have been strictly reviewed by the study members, and all the data are true, reliable and repeatable. The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics. CA Cancer J. Clin. 71(1), 7–33 (2021).
Expert consensus on diagnosis and treatment of chemotherapy-induced thrombocytopenia in cancer patients (2014). Zhonghua zhong liu za zhi Chin. J. Oncol. 36(11):876–879.
Epstein, R. S. et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: Results from an online survey of patients with solid tumors. Adv. Ther. 37(8), 3606–3618 (2020).
Wu, Y., Aravind, S., Ranganathan, G., Martin, A. & Nalysnyk, L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: A descriptive study of a large outpatient oncology practice database, 2000–2007. Clin. Ther. 31(Pt 2), 2416–2432 (2009).
Hitron, A. et al. Incidence and risk factors of clinically significant chemotherapy-induced thrombocytopenia in patients with solid tumors. J. Oncol. Pharm. Pract. 17(4), 312–319 (2011).
Elting, L. S. et al. Cost of chemotherapy-induced thrombocytopenia among patients with lymphoma or solid tumors. Cancer 97(6), 1541–1550 (2003).
Treatment patterns and outcomes in management of solid cancer patients suffering from thrombocytopenia in Penang hospital. Asian. Pac. J. Cancer Prev. 12(11):2841–5. (2011)
Lyman, G. H. Impact of chemotherapy dose intensity on cancer patient outcomes. J. Nat. Compr. Cancer Netw. 7, 1 (2009).
Denduluri, N. et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J. Nat. Compr. Cancer Netw. JNCCN. 13(11), 1383–1393 (2015).
Elting, L. S. et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J. Clin. Oncol. Official J Am. Soc. Clin. Oncol. 19(4), 1137–1146 (2001).
Blumberg, N., Heal, J. M., Phillips, G. L. & Phipps, R. P. Platelets—to transfuse or not to transfuse. The Lancet. 380(9850), 1287–1289 (2012).
Khorana, A. A. et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch. Int. Med. 168(21), 2377–2381 (2008).
Tepler, I. et al. A randomized placebo-controlled trial of recombinant human__interleukin-11 in cancer patients with severe__thrombocytopenia due to chemotherapy. Blood 87, 3607–3614 (1996).
Jiang, Xu. J. F. R. & Mugelli, A. Age-Dependent Atrial Remodeling Induced by Recombinant__Human Interleukin-11_ Implications for Atrial Flutter_Fibrillation™. J. Cardiovasc. Pharmacol. 39, 435–440 (2002).
Li, S. The application analysis of recombinant human interleu akin 11 in the treatment of chemotherapy induced thrombocytopenia. Chin. Hosp. Pharm. J. 38(14), 1530–1544 (2018).
Ma, W. Heart failure induced by recombinant human interleukin 11 for injection: A case report. Zhongguo yao wu jing jie. 13(10), 635–636 (2016).
Xu, J. Age-dependent atrial remodeling induced by recombinant__ human interleukin-11_ Implications for atrial flutter_ fibrillation. J. Cardiovasc. Pharmacol. 39(3), 435–440 (2002).
HUA bao-lai Zy-q. Development of recombinant human thrombopotietin (2005) Anitbodies following multi-dosing, subcutaneously medication in thrompocytopenic patients. Chin. J. Thrombosis Hemostasis. 11.
Shaw, J. L., Nielson, C. M., Park, J. K., Marongiu, A. & Soff, G. A. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur. J. Haematol. 106(5), 662–672 (2021).
Weycker, D. et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer 19(1), 151 (2019).
Zhu, C. et al. Profiling chemotherapy-associated myelotoxicity among Chinese gastric cancer population receiving cytotoxic conventional regimens: Epidemiological features, timing, predictors and clinical impacts. J. Cancer. 8(13), 2614–2625 (2017).
Tanriverdi, O. Predictive value of baseline plasma D-dimers for chemotherapy- induced thrombocytopenia in patients with stage III colon cancer: A pilot study. Asian. Pac. J. Cancer Prev. 14(1), 293–297 (2013).
Razzaghdoust, A., Mofid, B. & Zangeneh, M. Predicting chemotherapy-induced thrombocytopenia in cancer patients with solid tumors or lymphoma. J. Oncol. Pharm. Pract. 26(3), 587–594 (2020).
Kilpatrick, K. et al. Occurrence and management of thrombocytopenia in metastatic colorectal cancer patients receiving chemotherapy: Secondary analysis of data from prospective clinical trials. Clin. Colorectal Cancer 20(2), 170–176 (2021).
Jiang, Z. et al. Comparison of S-1 plus oxaliplatin (SOX) and capecitabine plus oxaliplatin (XELOX) as adjuvant chemotherapies for stage II and III gastric cancer after D2 resection: A single-center retrospective study. Asia Pac. J. Clin. Oncol. 16(3), 180–186 (2020).
Paz-Ares, L. et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3, randomised controlled trial. Lancet Oncol. 13(3), 247–255 (2012).
Reck, M. et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 27(8), 1227–1234 (2009).
Zhou, C. et al. Beyond: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J. Clin Oncol. Off. J. Am. Soc. Clin. Oncol. 33(19), 2197–2204 (2015).
Ohe, Y. et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm cooperative study in Japan. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 18(2), 317–323 (2007).
Fossella, F. et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 21(16), 3016–3024 (2003).
Andre, T. et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 350(23), 2343–2351 (2004).
Schmoll, H. J. et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J. Clin. Loncol. Off. J. Am.Soc. Clin. Oncol. 25(1), 102–109 (2007).
Conroy, T. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379(25), 2395–2406 (2018).
Uesaka, K. et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). The Lancet. 388(10041), 248–257 (2016).
Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. The Lancet. 389(10073), 1011–1024 (2017).
Ten Berg, M. J. et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: Results from a retrospective hospital-based cohort study. Drug. Saf. 34(12), 1151–1160 (2011).
Scheithauer, W. et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: Safety results of a randomized, phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 14(12), 1735–1743 (2003).
Li, L., Jiang, W. & Wang, J. Clinical analysis of 275 cases of acute drug-induced liver disease. Front. Med. China. 1(1), 58–61 (2007).
Vigano, L. et al. Liver resection for colorectal metastases after chemotherapy: Impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann. Surg. 258(5), 731–740 (2013).
Peppercorn RHR, P. D., Wilson, P., Slevin, M. L. & Gupta, R. K. (1998) Demonstration of hepatic steatosis by computerized__tomography in patients receiving 5-fluorouracil-based__therapy for advanced colorectal cancer. Br. J. Cancer. 77, 11 (2008).
Rubbia-Brandt, L. et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Annals Oncol Off. J Eur. Soc. Med. Oncol. 15(3), 460–466 (2004).
Vauthey, J. N. et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J. Clin. Oncol. Off. J. Am. Soc. of Clin. Oncol. 24(13), 2065–2072 (2006).
Author information
Authors and Affiliations
Contributions
S.Z. and J.F. contributed to the conception of the study. S.Z. and B.S. wrote the manuscript. B.S. and J.F. performed the data analyses. S.Z. and B.S. contributed the same to the article and as the co-first authors. C.L. contributed significantly to analysis and manuscript preparation. W.T., X.Z., X.J., X.X., Q.W., and H.Z. helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, S., Song, B., Li, C. et al. The predictive model for risk of chemotherapy-induced thrombocytopenia based on antineoplastic drugs for solid tumors in eastern China. Sci Rep 13, 3185 (2023). https://doi.org/10.1038/s41598-023-27824-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27824-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.