Abstract
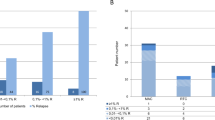
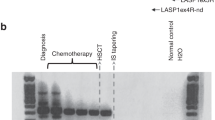
The role of chimerism analysis as a prognostic indicator of relapse after hematopoietic stem cell transplantation (SCT) is controversial. We monitored chimerism status by short tandem repeat-based polymerase chain reaction (PCR) in T- and non-T-cell subsets and retrospectively evaluated clinical outcome in 96 patients with acute myeloid leukemia after myeloablative (MA) or reduced-intensity conditioning SCT. Fifty-six percent of 80 patients in the MA group demonstrated complete donor chimerism (CC) at all time points, whereas 6% had decreasing mixed chimerism (MC), 8% stable MC, 25% increasing MC and 3% increasing and decreasing MC. In 16 RIC patients, these percentages were 12, 50, 6, 6 and 19, respectively, together with 6% nonengraftment. Forty-three out of 96 patients experienced relapse. The last chimerism evaluation before relapse revealed increasing MC in only eight patients. In samples taken between 1 and 6 months post SCT, CC/decreasing MC was significantly related with a lower risk of relapse (31 versus 83%, P<0.000) and mortality (38 versus 83%, P<0.000) than with MC/increasing MC. However, the development of relapse was very rapid. Only very frequent monitoring of chimerism status by highly sensitive methods might identify impending relapse and allow early immunological intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Durnam DM, Anders KR, Fisher L, O'Quigley J, Bryant EM, Thomas ED . Analysis of the origin of marrow cells in bone marrow transplant recipients using a Y-chromosome-specific in situ hybridization assay. Blood 1989; 74: 2220–2226.
Wessman M, Popp S, Ruutu T, Volin L, Cremer T, Knuutila S . Detection of residual host cells after bone marrow transplantation using non-isotopic in situ hybridization and karyotype analysis. Bone Marrow Transplant 1993; 11: 279–284.
Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2001; 7: 473–485.
Acquaviva C, Duval M, Mirebeau D, Bertin R, Cave H . Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Paris-Robert Debre experience. Leukemia 2003; 17: 241–246.
Chalandon Y, Vischer S, Helg C, Chapuis B, Roosnek E . Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Geneva experience. Leukemia 2003; 17: 228–231.
Hancock JP, Goulden NJ, Oakhill A, Steward CG . Quantitative analysis of chimerism after allogeneic bone marrow transplantation using immunomagnetic selection and fluorescent microsatellite PCR. Leukemia 2003; 17: 247–251.
Kreyenberg H, Holle W, Mohrle S, Niethammer D, Bader P . Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Tuebingen experience. Leukemia 2003; 17: 237–240.
Thiede C, Kellermann T, Schwerdtfeger R, Baurmann H, Steudel C, Ehninger G et al. Real-time PCR for the STY-gene allows sensitive and quantitative chimerism analysis after allogeneic blood stem cell transplantation: clinical results in 43 patients. Bone Marrow Transplant 2003; 31 (Suppl 1): S23; A181 (abstract).
Verdonck LF, Dekker AW, de Gast GC, van Kempen ML, Lokhorst HM, Nieuwenhuis HK . Allogeneic bone marrow transplantation with a fixed low number of T cells in the marrow graft. Blood 1994; 83: 3090–3096.
Thomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE et al. Bone-marrow transplantation (second of two parts). N Engl J Med 1975; 292: 895–902.
Miller SA, Dykes DD, Polesky HF . A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
de Weger RA, Tilanus MG, Scheidel KC, van den Tweel JG, Verdonck LF . Monitoring of residual disease and guided donor leucocyte infusion after allogeneic bone marrow transplantation by chimaerism analysis with short tandem repeats. Br J Haematol 2000; 110: 647–653.
Thiede C, Bornhauser M, Ehninger G . Strategies and clinical implications of chimerism diagnostics after allogeneic hematopoietic stem cell transplantation. Acta Haematol 2004; 112: 16–23.
Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T . How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant 2005; 35: 107–119.
Guimond M, Busque L, Baron C, Bonny Y, Belanger R, Mattioli J et al. Relapse after bone marrow transplantation: evidence for distinct immunological mechanisms between adult and paediatric populations. Br J Haematol 2000; 109: 130–137.
Barrios M, Jimenez-Velasco A, Roman-Gomez J, Madrigal ME, Castillejo JA, Torres A et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica 2003; 88: 801–810.
Choi SJ, Lee KH, Lee JH, Kim S, Chung HJ, Lee JS et al. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant 2000; 26: 327–332.
Mattsson J, Uzunel M, Tammik L, Aschan J, Ringden O . Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia 2001; 15: 1976–1985.
Miura Y, Tanaka J, Toubai T, Tsutsumi Y, Kato N, Hirate D et al. Analysis of donor-type chimerism in lineage-specific cell populations after allogeneic myeloablative and non-myeloablative stem cell transplantation. Bone Marrow Transplant 2006; 37: 837–843.
Najfeld V, Burnett W, Vlachos A, Scigliano E, Isola L, Fruchtman S . Interphase FISH analysis of sex-mismatched BMT utilizing dual color XY probes. Bone Marrow Transplant 1997; 19: 829–834.
Schaap N, Schattenberg A, Mensink E, Preijers F, Hillegers M, Knops R et al. Long-term follow-up of persisting mixed chimerism after partially T cell-depleted allogeneic stem cell transplantation. Leukemia 2002; 16: 13–21.
Wasch R, Bertz H, Kunzmann R, Finke J . Incidence of mixed chimaerism and clinical outcome in 101 patients after myeloablative conditioning regimens and allogeneic stem cell transplantation. Br J Haematol 2000; 109: 743–750.
Briones J, Urbano-Ispizua A, Lawler M, Rozman C, Gardiner N, Marin P et al. High frequency of donor chimerism after allogeneic transplantation of CD34+-selected peripheral blood cells. Exp Hematol 1998; 26: 415–420.
Roux E, Helg C, Dumont-Girard F, Chapuis B, Jeannet M, Roosnek E . Analysis of T-cell repopulation after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts. Blood 1996; 87: 3984–3992.
Socie G, Cayuela JM, Raynal B, Esperou H, Fund X, Raffoux C et al. Influence of CD34 cell selection on the incidence of mixed chimaerism and minimal residual disease after allogeneic unrelated donor transplantation. Leukemia 1998; 12: 1440–1446.
Fernandez-Aviles F, Urbano-Ispizua A, Aymerich M, Colomer D, Rovira M, Martinez C et al. Serial quantification of lymphoid and myeloid mixed chimerism using multiplex PCR amplification of short tandem repeat-markers predicts graft rejection and relapse, respectively, after allogeneic transplantation of CD34+ selected cells from peripheral blood. Leukemia 2003; 17: 613–620.
Baron F, Little MT, Storb R . Kinetics of engraftment following allogeneic hematopoietic cell transplantation with reduced-intensity or nonmyeloablative conditioning. Blood Rev 2005; 19: 153–164.
Mattsson J, Uzunel M, Brune M, Hentschke P, Barkholt L, Stierner U et al. Mixed chimaerism is common at the time of acute graft-versus-host disease and disease response in patients receiving non-myeloablative conditioning and allogeneic stem cell transplantation. Br J Haematol 2001; 115: 935–944.
Baron F, Baker JE, Storb R, Gooley TA, Sandmaier BM, Maris MB et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2004; 104: 2254–2262.
Keil F, Prinz E, Moser K, Mannhalter C, Kalhs P, Worel N et al. Rapid establishment of long-term culture-initiating cells of donor origin after nonmyeloablative allogeneic hematopoietic stem-cell transplantation, and significant prognostic impact of donor T-cell chimerism on stable engraftment and progression-free survival. Transplantation 2003; 76: 230–236.
Uzunel M, Mattsson J, Brune M, Johansson JE, Aschan J, Ringden O . Kinetics of minimal residual disease and chimerism in patients with chronic myeloid leukemia after nonmyeloablative conditioning and allogeneic stem cell transplantation. Blood 2003; 101: 469–472.
Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood 1999; 94: 3234–3241.
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005; 23: 1993–2003.
Michallet AS, Furst S, Le QH, Dubois V, Praire A, Nicolini F et al. Impact of chimaerism analysis and kinetics on allogeneic haematopoietic stem cell transplantation outcome after conventional and reduced-intensity conditioning regimens. Br J Haematol 2005; 128: 676–689.
Acknowledgements
We thank Dr P Westers, statistician at the Center for Biostatistics, for his help in the statistical analysis of this study. We are also grateful to Dr E Meijer, M Gerrits and J van der Giessen (Department of Hematology, University Medical Center Utrecht) for providing data. J van Loon and WTM van Blokland are gratefully acknowledged for their contribution in the STR analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huisman, C., de Weger, R., de Vries, L. et al. Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant 39, 285–291 (2007). https://doi.org/10.1038/sj.bmt.1705582
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705582
Keywords
This article is cited by
-
Lineage-specific early complete donor chimerism and risk of relapse after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia
Bone Marrow Transplantation (2022)
-
Evaluation of EuroFlow minimal residual disease measurement and donor chimerism monitoring following tandem auto-allogeneic transplantation for multiple myeloma
Bone Marrow Transplantation (2021)
-
Phase I trial of maintenance selinexor after allogeneic hematopoietic stem cell transplantation for patients with acute myeloid leukemia and myelodysplastic syndrome
Bone Marrow Transplantation (2020)
-
Quantitative chimerism in CD3-negative mononuclear cells predicts prognosis in acute myeloid leukemia patients after hematopoietic stem cell transplantation
Leukemia (2020)
-
Kinetics of peripheral blood chimerism for surveillance of patients with leukemia and chronic myeloid malignancies after reduced-intensity conditioning allogeneic hematopoietic SCT
Bone Marrow Transplantation (2015)