Abstract
Objective:
To analyze factors with impact on the functional outcome for patients with surgically treated intramedullary spinal cord tumors (IMSCT) and to point out characteristics of the different histological entities.
Setting:
Neurosurgical Department, University of Essen, Germany.
Methods:
Between 1990 and 2000, a consecutive series of 78 patients were referred to our institution and underwent surgical treatment. There were 46 (59%) male and 32 (41%) female patients. Mean age was 43.3 years. Functional outcome was analyzed depending on histological features, age, tumor localization and the extension of involved spinal segments. The mean follow-up period was 34.4 months. Operative removal of the IMSCT was performed under standard microsurgical conditions with intraoperative monitoring of somatosensory-evoked potentials (SSEP).
Results:
The most frequently involved localization was the cervical and cervicothoracic region (55%) followed by the thoracic region (32%) and the medullar conus (13%). The most frequent IMSCTs were neuroepithelial tumors in 44 patients (56.5%) including 32 patients with ependymomas, 15 astrocytomas, and two lesions without further histological classification. Non-neuroepithelial tumors included 10 metastases, nine cavernomas, eight hemangioblastomas, one dermoidal cyst and one enterogenetic cyst. Complete tumor removal was achieved in 65 cases (83.3%) and subtotal resection in nine cases. In four cases a biopsy was performed only. The overall postoperative neurological state was improved or unchanged in 51 patients (65.4%) and worsened in 27 patients (34.6%). A favorable functional outcome was observed in 94.1% of patients with vascular tumors, in 61.3% of patients with low-grade neuroepithelial tumors and in 53.3% of patients with malignant tumors.
Conclusions:
The strongest predicting factor of functional outcome was the preoperative neurological condition beyond the histological differentiation of the IMSCT. Although there was no outcome difference with respect to the age and tumor extension, thoracically located IMSCTs proved to harbor an increased risk of postoperative surgical morbidity.
Similar content being viewed by others
Introduction
In the era before introduction of modern neuroimaging tools, usually spinal myelography was the diagnostic method of choice to detect spinal cord lesions. However, diagnosis remained difficult and demonstration of the true localization and extension of intramedullary tumors was often only achieved by explorative surgical exposure. Nowadays spinal magnetic resonance imaging (MRI) and, if necessary, angiography are applied routinely in the diagnosis of intramedullary spinal cord tumors (IMSCT), leading to a better understanding of the variability, for both, intramedullary and extramedullary lesions. With the additional improvement of microsurgical procedures, complete removal of IMSCTs with satisfactory functional results has been reported.1,2,3,4,5,6,7,8,9,10,11,12,13 Thus, surgery advanced to be the primary option in the treatment of IMSCTs.
This study was conducted to analyze factors with impact on the functional outcome in a series of 78 surgically treated patients with IMSCTs and to point out the characteristics of the different histological entities.
Patients and methods
Between 1990 and 2000, a total of 78 patients with an IMSCT were referred to our institution and underwent surgical treatment. There were 46 males (59%) and 32 females (41%). Mean age was 43.3 years (range 1–75 years). Basic demographic data, clinical and radiological presentation and intraoperative observations were evaluated. Neuroepithelial tumors were classified as low-grade tumors (WHO grade I and II) and high-grade tumors (WHO grade III and IV). The patient's pre- and postoperative neurological state was classified according to the Frankel scale14 in order to achieve a grading of functional disturbance of daily life activities and gait disturbances. According to the Frankel grade, outcome was classified as poor (A+B), fair (C), and good (D+E). The functional outcome was analyzed depending on histological features, tumor localization and extension of the tumor. With regard to tumor extension, lesions were classified into two groups, those involving one–three spinal segments, and those involving more than three spinal segments. Preoperative neuroimaging included MRI in all cases. Spinal angiography was performed, if MRI revealed a vascular lesion. All patients were followed up clinically and by spinal MRI. The mean follow-up period was 34.4 months (range 1–109 months). Operative removal of the IMSCT was performed under standard microsurgical conditions with intraoperative monitoring of somatosensory-evoked potentials (SSEP). Tumor-associated cysts were generally resected including the cyst wall in cases with suspicion of tumor infiltration; however, in patients with hemangioblastomas the tumor cyst was not removed. Tumor removal was classified as complete, incomplete or biopsy according to intraoperative observations and postoperative MRI. A more detailed subdivision was avoided because a precise validation of total, 99% or 80–95% tumor removal was regarded as unsatisfactory. Recurrence-free survival was evaluated using the Kaplan–Meier analysis.
Recurrence-free period was defined as the interval between surgery and the last follow-up without clinical and radiological evidence tumor regrowth.
Statistical analyses were performed using SPSS (SPSS Inc., version 11.0). Crosstables were calculated and χ2-test was performed to assess the impact of different variables on outcome. Functional outcome was dichotomized into categories ‘good’ versus ‘fair and poor’.
The most frequently involved localization was the cervical and cervicothoracic region followed by the thoracic region and the medullar conus. An overview is given in Table 1.
The most frequent IMSCTs were low-grade neuroepithelial tumors including ependymomas and astrocytomas in 44 patients (56.5%). In two of these patients with a neuroepithelial tumor, further histological differentiation was not obtainable. The most frequent non-neuroepithelial tumors were metastases including seven metastases of adenocarcinomas and three metastases of sarcomas. The histological typing is summarized in Table 2.
Results
Surgery
Surgery was performed in a semisitting position if the tumor was located in the upper and middle cervical region and in a prone position for lesions of the lower cervical and thoracic region or the medullar conus.
Laminectomy was performed in 35 cases and osteoplastic laminotomy with reconstruction of the posterior spinal column15 was performed in 43 patients. In four of the latter cases, an expansive laminoplasty for decompression was performed because tumor removal proved to be impossible due to a diffuse infiltration of the spinal cord.
In 65 cases (83.3%), the IMSCT was removed completely and in nine cases incompletely (four metastases, three low-grade ependymomas, one high-grade astrocytoma, one enterogenetic cyst). Owing to the absence of a clear plane of dissection in four cases (5.1%), a biopsy was performed only (three low-grade astrocytomas, one metastasis).
Dexamethasone was administered preoperatively in cases with acute neurological deterioration or MRI signs of edema of the surrounding spinal cord tissue. Postoperatively all patients received routine administration of dexamethasone.
Complications
In the early postoperative course we observed CSF leaks in two patients. One of these patients had to be reoperated. Another patient developed a meningitis treated successfully with systemic antibiotics.
Outcome
Compared to the preoperative neurological condition, the overall postoperative state at last follow-up was improved or unchanged in 65.4% patients (n=51) and worse in 34.6% patients (n=27). The preoperative and postoperative Frankel grades are shown in Table 3.
With respect to histological classification and pathological grading, we observed varying functional outcomes. An overview is given in Table 4. Statistical analysis identified the preoperative neurological state to be a significant predictor of functional outcome in low-grade neuroepithelial tumors. The relationship between other variables and outcome did not reach the level of statistical significance.
Low-grade neuroepithelial tumors
Patients with low-grade neuroepithelial tumors showed a good functional outcome in 61.4% (n=27) and a fair outcome in 27.3% (n=12). In all, 11.3% (n=5) showed a poor functional outcome with significant postoperative neurological deficits (all Frankel grade B). There was no outcome difference depending on age with a good functional outcome in 72% for patients ⩽40 years (n=13) and 67% for patients >40 years (n=14). A good functional outcome was observed for tumors of the cervical region in 62.5% (n=10), the cervicothoracic region in 66.6% (n=8), the medullar conus in 75% (n=3), but only in 50% for tumors located in the thoracic region (n=6). Tumors extending by more than three segments showed a good functional outcome in 69% (n=16) and those involving only up to three segments only in 52% (n=11). 69.2% of the patients with a good preoperative neurological state remained good postoperatively.
The two largest histological subgroups, low-grade ependymomas (n=30) and low-grade astrocytomas (n=12) showed the following features.
Ependymomas grade I+II
For patients with low-grade ependymomas, we observed no outcome differences depending on tumor extension. In 16 of 30 patients, the postoperative functional state was unchanged compared to the preoperative condition and in 11 patients the functional grade deteriorated by one grade. The worst functional outcome (Frankel grade B) was observed in three of nine patients with a thoracically located ependymoma. In two of these patients, the clinical state deteriorated by two grades leading to significant functional disturbances.
Astrocytomas grade I+II
Whereas patients with low-grade ependymomas showed an equal regional distribution of their tumors, in patients with low-grade astrocytomas, we observed a predilection for the cervical and cervicothoracic region (seven of 12 patients). There was no outcome difference with respect to tumor extension. In eight of 12 patients (67%) the postoperative state was unchanged compared to the preoperative state, whereas in four patients, the functional state deteriorated by one grade. In two of these four patients, neurological deterioration was not due to the surgical procedure, but caused by a progressive infiltrative tumor growth. In these two cases, only biopsy was performed for histological examination without postoperative neurological deterioration. Similar to the observation in low-grade ependymomas, the worst postoperative state in this group (Frankel grade B) was observed in a patient with a thoracically located lesion.
Vascular tumors
The most favorable functional results were seen in patients with vascular tumors. In all, 94.1% (16 of 17 patients) showed a good functional outcome with no or only slight neurological deficits and all were able to walk independently (Frankel grades D and E). All patients with a good preoperative neurological condition (n=13) remained good postoperatively. Of the four patients with a fair preoperative state three improved postoperatively and one patient remained unchanged. There was no outcome difference depending on age or tumor localization. None of the vascular tumors involved more than three spinal segments and all were removed completely.
High-grade neuroepithelial tumors and metastases
In contrast, the outcome for high-grade tumors (metastases n=10, astrocytomas grade III n=3 and ependymomas grade III n=2) was worse with only 53% (n=8) in a good postoperative condition and 33.3% (n=5) in a fair condition with preserved motor and sensory function but inability to walk independently. There was no outcome difference depending on age, tumor localization or tumor extension for high-grade neuroepithelial tumors.
Recurrence rate
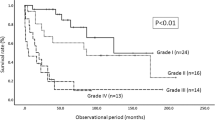
According to a Kaplan–Meier analysis we observed a recurrence rate of 9% within 5 years postoperatively for patients with neuroepithelial tumors (Figure 1). We observed no tumor recurrence in patients with vascular tumors. Six patients died due to progression of their systemic malignant disease during the observation period.
Discussion
With the introduction of modern neuroimaging methods and the development of microsurgical techniques, reports of surgically treated IMSCT became more apparent in the literature.1,8 These technical advances, especially the use of the Cavitron ultrasonic aspirator and the routine use of SSEPs for intraoperative monitoring,16,17,18,19,20 contributed to the safety of the procedure. Illustrative cases are presented in Figures 2 and 3.
(a) (Case No. 16) This 28-year-old female patient suffered from a neurofibromatosis type 2. Cranial MRI revealed bilateral acoustic schwannomas and multiple intracranial meningiomas. Preoperative spinal MRI showed an intramedullary low-grade ependymoma extending from C5–T6 with homogeneous gadolinium enhancement on T1-weighted images with a cranially located tumor cyst (black arrow) and a sharply defined margin between the tumor and the surrounding neural tissue (white arrow). On the right side is the T2-weighted image showing the extending medullar edema (arrow). (b) After a cervicothoracic osteoplastic laminotomy and dural incision, the markedly swollen spinal cord was exposed. Note the clearly defined plane of cleavage between normal neural tissue and tumor (arrows) after midline myelotomy. (c) Postoperative gadolinium-enhanced T1-weighted (left) and T2-weighted MRI scan (right) shows no residual tumor. The patient was followed up closely by clinical examinations and MRI for 3 years and 9 months without evidence of recurrence of the spinal tumor or progression of her intracranial tumors
MR images pre- and postoperatively (left side) demonstrate a cystic ependymoma WHO II extending from C1 to C7 with a nodular enhancement at C2 (arrow) which was completely resected via an osteoplastic laminotomy. Intraoperative recordings of M-SEP (right side) remained completely stable during the surgical procedure. This case demonstrates that M-SEP are not necessarily affected by a carefully performed midline myelotomy and that a stable SEP recording may encourage the surgeon to proceed with radical tumor resection
Laminectomy versus osteoplastic laminotomy
As mentioned above, laminectomy was performed in 35 patients and osteoplastic laminotomy with reconstruction of the posterior spinal column in 43 patients. In contrast to Crisante and Herrmann,21 who did not observe postoperative spinal deformities or the development of spinal problems in his pediatric patients, in our experience, osteoplastic laminectomy so far seems not to reduce the risk of spinal deformities compared to patients who underwent laminectomy only. The impact of these procedures on the prevention of spinal deformities and its influence on the development of postoperative pain is currently under investigation at this institution. Our current surgical strategy consists of reconstruction of the posterior spinal column and refixation of the laminotomy bloc with microplates.15
Tumor resectability
Although several authors reported a series with complete tumor removal and good postoperative functional outcome,4,5,22 others pointed out the limitations of the surgery caused by the absence of a clear plane of cleavage, resulting in partial resection, decompression or biopsy only for diagnosis.23,24 Particularly astrocytomas are characterized by an infiltrative growth, often leading to incomplete tumor resection.24,25,26,27 Ependymomas are generally recognized as resectable lesions due to a clearly defined plane of dissection.5,6,9,11 In our experience, complete tumor removal was possible in 83.3% (n=65) whereas in 11.5% (n=9) the tumor only was removed subtotally. Owing to the absence of a clear plane of dissection and to avoid additional medullar damage with irreversible neurological deterioration in 5.1% (n=4), a biopsy was performed only. In six of these 13 cases with incomplete removal, resection was limited due to an infiltrative malignant tumor growth and in seven cases caused by an infiltrative low-grade neuroepithelial tumor including one patient with an enterogenetic cyst. However, we observed no significant differences regarding respectability for the neuroepithelial subtypes. Our rate for complete removal was 90% for patients with ependymomas and 87% for patients with astrocytomas. Our rate for complete removal of 90% for patients with ependymomas is similar to those reported in the literatures.5,9,12 In contrast, the reported rates for complete resection of low-grade astrocytomas varies between 5 and 67%.1,2,3,12,16,21,25,26,27 In 1992, Epstein et al4 reported a series of 25 patients undergoing gross total resection for intramedullary low-grade astrocytoma in all cases. In our opinion, this widespread variation of the extend of resection is a result of both, the refinement of surgical techniques17,28 and the surgeon's attitude. The importance of a varying aggressiveness of surgery is underlined by the report of Epstein et al5 in 1993. In his series of 38 patients with intramedullary spinal cord ependymomas, all but one patient underwent complete resection, although 14 of these patients had underwent surgery elsewhere prior to definitive treatment. Of these, nine patients had underwent subtotal resection previously and five patients had only a biopsy. None of these 38 patients had a tumor recurrence after a mean follow-up period of 24 months.
The gratifying functional results for vascular tumors are underlined by the fact that total removal was achieved in all cases. Although there are differences between cavernomas and hemangioblastomas concerning the operative strategy, both are characterized by clear plane of dissection facilitating removal.
Functional outcome
The strongest predicting factor of functional outcome was the preoperative neurological condition, as shown for patients with neuroepithelial tumors. In 78.5% of the patients with good initial condition (51 of 65 patients) the postoperative state remained good (Frankel grade D or E). Surprisingly, patients with low-grade neuroepithelial tumors and tumor extension of more than three spinal segments showed a good postoperative functional state in 69.6%, whereas patients with a tumor extension of less than three segments showed a good functional outcome only in 52%. This observation can be explained by the fact that tumors extending by more than three segments were mostly ependymomas, and as mentioned above, characterized by a clearer-defined plane of dissection compared to astrocytomas. Further analysis showed no other factors with significant impact on the functional outcome. In the literature other factors were identified to effect outcome after surgery. In his analysis of 36 cases, Hoshimaru et al9 recently reported that the most important predicting factor for surgical morbidity was the intraoperative observation of arachnoid scarring and spinal cord atrophy. A similar observation was made by Samii and Klekamp,12 reporting a worse surgical outcome for patients who presented with arachnoid scarring at surgery and pointing out that particularly astrocytomas are more likely to present with arachnoid scarring. On the other hand some authors reported that tumor localization has some impact on surgical outcome. Especially, IMSCTs of the thoracic region are reported to harbor an increased risk of surgical morbidity.9,21
In accordance to these reports we made a similar observation for thoracically located tumors. The worst functional results (Frankel grade B) were observed in three of the 30 patients with low-grade ependymomas and in one of the 12 patients with a low-grade astrocytoma. In all four cases, the tumor was located exclusively in the thoracic cord, with a deterioration of two Frankel grades in two of these four patients. A detailed analysis of these four patients revealed no further risk factors such as significant spinal cord atrophy or arachnoid scarring. However, in our opinion the presence of spinal cord atrophy is difficult to determine. The decreased amount of tumor surrounding spinal cord tissue is caused by a mostly centromedullary extending tumor growth and its diameter is highly correlated with tumor size and extension. Therefore, we were cautious to determine this phenomenon as the presence of atrophy as known in degenerative spinal cord diseases.
Summarizing our experience, the postoperative outcome is closely correlated to the histological type and the grade of infiltrative tumor growth. Thoracically located lesions seem to harbor an increased risk of surgical morbidity. Nevertheless, according to others,1,2,9,21,25 the most important predicting factor for postoperative outcome is the preoperative neurological condition. This observation suggests that operative treatment should be performed in an early stage of the disease.
Adjuvant radiotherapy and recurrence rate
Surgery is generally considered as the primary treatment for IMSCTs. In contrast, postoperative management is still controversial and inconsistent, especially for those patients with low-grade tumors. In 1989, Cooper3 presented a series of 51 patients operated on in a period of 7 years. In his opinion, postoperative radiation therapy is indicated for all patients with astrocytomas, irrespective of grading and extent of tumor removal, as well as for incompletely removed ependymomas. In a more recent study, Kane et al23 published a series of 54 patients with surgically treated IMSCTs reporting that adjuvant postoperative radiotherapy was not used routinely if complete tumor removal had been achieved, for both, ependymomas and astrocytomas. Furthermore, he described that patients with postoperative radiotherapy experienced a similar outcome compared to those patients without adjuvant treatment, concluding that the effectiveness of radiotherapy remains unclear and has yet to be demonstrated. In the presented series, postoperative radiotherapy was performed only in cases with high-grade IMSCTs. Only two of our patients with low-grade neuroepithelial tumors and complete tumor resection experienced a tumor recurrence. This low recurrence rate in our opinion does not justify an aggressive postoperative radiation therapy. Furthermore, three of the five patients with high-grade neuroepithelial tumors showed no tumor recurrence during a follow-up period of 4, 8 and 9 years, respectively. To compare these data with the literature appears difficult, because reports of anaplastic intramedullary ependymomas are extremely rare. Hoshimaru's series included only one case out of 36 patients.9 This patient underwent subtotal resection leading to progressive neurological deterioration after a follow-up period of 49 months. Fornari et al25 reported on 31 ependymomas, including four patients with anaplastic ependymomas. Of these, two patients experienced tumor regrowth within 2 and 3 years, respectively. However, most reports missed to define further differentiation or grading of the reported intramedullary ependymomas.2,3,5,9,12,21,23
We think that this favorable long-term outcome is rather a result of radical tumor resection than of adjuvant postoperative radiation and that the crucial factor for tumor recurrence is the extent of surgical tumor resection. Once the tumor is removed completely, close clinical and radiological examination is necessary to detect tumor regrowth at an early stage. Adjuvant radiation treatment should be preserved for malignant tumors or nonresectable low-grade tumors with clinical and radiological evidence of progression.
References
Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ . Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg (Spine) 2000; 93: 183–193.
Cooper PR, Epstein F . Radical resection of intramedullary spinal cord tumours in adults. Recent experience in 29 patients. J Neurosurg 1985; 63: 492–499.
Cooper PR . Outcome after operative treatment of intramedullary spinal cord tumours in adults: intermediate and long-term results in 51 patients. Neurosurgery 1989; 25: 855–859.
Epstein FJ, Farmer JP, Freed D . Adult intramedullary astrocytomas of the spinal cord. J Neurosurg 1992; 77: 355–359.
Epstein FJ, Farmer JP, Freed D . Adult intramedullary spinal cord ependymomas: the results of surgery in 38 patients. J Neurosurg 1993; 79: 204–209.
Ferrante L, Mastronardi L, Celli P, Lunardi P, Acqui M, Fortuna A . Intramedullary spinal cord ependymomas – a study of 45 cases with long-term follow-up. Acta Neurochir (Wien) 1992; 119: 74–79.
Greenwood JJ . Intramedullary tumors of the spinal cord. A follow-up study after total surgical removal. J Neurosurg 1963; 20: 665–668.
Hejazi N, Hassler W . Microsurgical treatment of intramedullary spinal cord tumors. Neurol Med Chir (Tokyo) 1998; 38: 266–273.
Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H . Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery 1999; 44: 264–269.
Iwasaki Y, Hida K, Sawamura Y, Abe H . Spinal intramedullary ependymomas: surgical results and immunohistochemical analysis of tumour proliferation activity. Br J Neurosurg 2000; 14: 331–336.
McCormick PC, Stein BM . Intramedullary tumors in adults. In: Stein BM, McCormick PC (eds). Neurosurgery Clinics in North America: Intradural Spinal Surgery, Vol. 1. WB Saunders Co: Philadelphia, 1990 pp 609–630.
Samii M, Klekamp J . Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery 1994; 35: 673–865.
Stein B . Surgery of intramedullary spinal cord tumors. Clin Neurosurg 1979; 26: 529–542.
Frankel HL et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 1969; 7: 179–192.
Wiedemayer H, Schoch B, Stolke D . Osteoplastic laminotomy using titanium microplates for reconstruction of the laminar roof: a technical note. Neurosurg Rev 1998; 21: 93–97.
Brotchi J et al. A survey of 65 tumors within the spinal cord: surgical results and the importance of preoperative magnetic resonance imaging. Neurosurgery 1991; 29: 651–656.
Epstein FJ . The Cavitron ultrasonic aspirator in tumor surgery. Clin Neurosurg 1983; 31: 497–505.
Epstein FJ, Farmer JP, Schneider SJ . Intraoperative ultrasonography: an important adjunct for intramedullary tumors. J Neurosurg 1991; 74: 729–733.
Kothbauer K, Deletis V, Epstein F . Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg 1997; 26: 247–254.
Wiedemayer H, Fauser B, Sandalcioglu IE, Schäfer H, Stolke D . The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg 2002; 96: 255–262.
Crisante L, Herrmann HD . Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery 1994; 35: 69–76.
McCormick PC, Torres R, Post KD, Stein BM . Intramedullary ependymoma of the spinal cord. J Neurosurg 1990; 72: 523–532.
Kane PJ, El-Mahdy W, Singh A, Powell MP, Crockard HA . Spinal intradural tumours: Part II – intramedullary. Br J Neurosurg 1999; 13: 558–563.
Sandalcioglu IE, Gasser Th, Wiedemayer H, Horsch S, Stolke D . Favourable outcome after biopsy and decompression of a holocord intramedullary spinal cord astrocytoma in a newborn. Eur J Paediatr Neurol 2002; 6: 179–182.
Fornari M et al. Microsurgical treatment of intramedullary spinal cord tumours. Acta Neurochir Suppl (Wien) 1988; 43: 3–8.
Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM . Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg 1995; 83: 590–595.
Sandler HM, Papadopoulos SM, Thornton AF, Ross DA . Spinal cord astrocytomas: results of therapy. Neurosurgery 1992; 40: 490–493.
Brotchi J . Intrinsic spinal cord tumor resection. Neurosurgery 2002; 50: 1059–1063.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sandalcioglu, I., Gasser, T., Asgari, S. et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord 43, 34–41 (2005). https://doi.org/10.1038/sj.sc.3101668
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101668
Keywords
This article is cited by
-
Pathogenesis of spinal intramedullary lipomas: two case reports
Journal of Medical Case Reports (2023)
-
The evolution of spinal cord surgery: history, people, instruments, and results
Child's Nervous System (2023)
-
The role of intraoperative extensor digitorum brevis muscle MEPs in spinal surgery
European Spine Journal (2023)
-
Dorsal column mapping in resection of intramedullary spinal cord tumors: a prospective comparison of two methods and neurological follow-up
Acta Neurochirurgica (2023)
-
Validity of magnetic resonance imaging (MRI) in the primary spinal cord tumors in routine clinical setting
Scientific Reports (2022)