Abstract
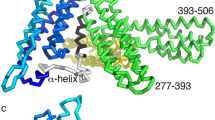
THE cadherins mediate cell adhesion and play a fundamental role in normal development1. They participate in the maintenance of proper cell–cell contacts: for example, reduced levels of epithelial cadherin (E-cadherin) correlate with increased invasiveness in many human tumour cell types2,3. The cadherins typically consist of five tandemly repeated extracellular domains, a single membrane-spanning segment and a cytoplasmic region4–6. The N-terminal extracellular domains mediate cell–cell contact7while the cytoplasmic region interacts with the cytoskeleton through the catenins8. Cadherins depend on calcium for their function: removal of calcium abolishes adhesive activity, renders cadherins vulnerable to proteases (reviewed in ref. 4) and, in E-cadherin, induces a dramatic reversible conformational change in the entire extracellular region9. We report here the X-ray crystal structure at 2.0 Å resolution of the two N-terminal extracellular domains of E-cadherin in the presence of calcium. The structure reveals a two-fold symmetric dimer, each molecule of which binds a contiguous array of three bridged calcium ions. Not only do the bound calcium ions linearize and rigidity the molecule, they promote dimerization. Although the N-terminal domain of each molecule in the dimer is aligned in a parallel orientation, the interactions between them differ significantly from those found in the neural cadherin (N-cadherin) N-terminal domain (NCD1) structure10. The E-cadherin dual-domain structure reported here defines the role played by calcium in the cadherin-mediated formation and maintenance of solid tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Takeichi, M. Science 251, 1451–1455 (1991).
Mareel, M., Bracke, M. & Van Roy, F. Molec. Biol. Rep. 19, 45–67 (1994).
Birchmeier, W. Bioessays 17, 97–99 (1995).
Takeichi, M. A. Rev. Biochem. 59, 237–252 (1990).
Blaschuk, O. W., Munroe, S. B. & Farookhi, R. Can. J. Oncol. 4, 291–301 (1994).
Overduin, M. et al. Science 267, 386–389 (1995).
Nose, A., Tsuji, K. & Takeichi, M. Cell 61, 147–155 (1990).
Kemler, R. Trends Genet. 9, 317–321 (1993).
Pokutta, S., Herrenknecht, K., Kemler, R. & Engel, J. Eur. J. Biochem. 223, 1019–1026 (1994).
Shapiro, L. et al. Nature 374, 327–337 (1995).
Tong, K. I. et al. FEBS Lett. 352, 318–322 (1994).
Ozawa, M., Engel, J. & Kemler, R. Cell 63, 1033–1038 (1990).
Brown, E. M., Vassilev, P. M. & Hebert, S. C. Cell 83, 679–682 (1995).
Ringwald, M. et al. EMBO J. 6, 3547–3653 (1987).
Overduin, M., Tong, K. I., Kay, C. M. & Ikura, M. J. Biomol. NMR. (in the press).
Oda, T. et al. Proc. natn. Acad. Sci. U.S.A. 91, 1858–1862 (1994).
Risinger, J. I., Berchuck, A., Kohler, M. F. & Boyd, J. J. Nature Genet. 7, 98–102 (1994).
Blaschuk, O. W., Sullivan, R., David, S. & Pouliot, Y. Devl Biol 139, 227–229 (1990).
Volberg, T., Geiger, B., Kartenbeck, J. & Franke, W. W. J. Cell Biol. 102, 1832–1842 (1986).
Kabsch, W. J. appl. Crystallogr. 21, 916–924 (1988).
Otwinowski, Z. in Proceedings of the CCP4 Study Weekend (eds Sawyer, L., Isaacs, N. & Bailey, S.) 56–62 (SERC Daresbury Laboratory, Warrington, UK, 1993).
Tesmer, J., Stemmler, T., Penner-Hahn, J., Davisson, V. & Smith, J. Proteins 18, 394–403 (1994).
Furey, W. & Swaminathan, S. Meth. Enzym. (in the press).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, Acta crystallogr. A 47, 110–119 (1991).
Brünger, A. X-PLOR, v3.1 A System for X-ray Crystallography and NMR (Yale University Press, New Haven, USA, 1992).
Evans, S. V. J. molec. Graph. 11, 134–138 (1993).
Kraulis, P. J. appl. Crystallogr. 24, 946–950 (1991).
Glusker, J. P. Adv. Prot. Chem. 42, 1–76 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagar, B., Overduin, M., Ikura, M. et al. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380, 360–364 (1996). https://doi.org/10.1038/380360a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/380360a0
This article is cited by
-
Molecular mechanism underlying the increased risk of colorectal cancer metastasis caused by single nucleotide polymorphisms in LI-cadherin gene
Scientific Reports (2023)
-
Study on the Interactions Between Cisplatin and Cadherin by Fluorescence Spectrometry and Atomic Force Microscopy
Journal of Fluorescence (2023)
-
Nucleation of cadherin clusters on cell-cell interfaces
Scientific Reports (2022)
-
Adhesion G protein-coupled receptor gluing action guides tissue development and disease
Journal of Molecular Medicine (2022)
-
Variants in CDH23 cause a broad spectrum of hearing loss: from non-syndromic to syndromic hearing loss as well as from congenital to age-related hearing loss
Human Genetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.