Abstract
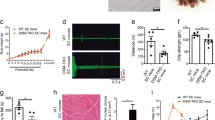
Expansion of a CTG trinucleotide repeat in the 3′ UTR of the gene DMPK at the DM1 locus on chromosome 19 causes myotonic dystrophy1,2,3, a dominantly inherited disease characterized by skeletal muscle dystrophy and myotonia, cataracts and cardiac conduction defects. Targeted deletion of Dm15, the mouse orthologue of human DMPK, produced mice with a mild myopathy4,5 and cardiac conduction abnormalities6, but without other features of myotonic dystrophy, such as myotonia and cataracts. We, and others, have demonstrated that repeat expansion decreases expression of the adjacent gene SIX5 (refs 7,8), which encodes a homeodomain transcription factor. To determine whether SIX5 deficiency contributes to the myotonic dystrophy phenotype, we disrupted mouse Six5 by replacing the first exon with a β-galactosidase reporter. Six5-mutant mice showed reporter expression in multiple tissues, including the developing lens. Homozygous mutant mice had no apparent abnormalities of skeletal muscle function, but developed lenticular opacities at a higher rate than controls. Our results suggest that SIX5 deficiency contributes to the cataract phenotype in myotonic dystrophy, and that myotonic dystrophy represents a multigenic disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brook, J.D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Fu, Y.H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Jansen, G. et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nature Genet. 13, 316–324 (1996).
Reddy, S. et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nature Genet. 13, 325–335 (1996).
Berul, C.I. et al. DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J. Clin. Invest. 103, R1–7 (1999).
Klesert, T.R., Otten, A.D., Bird, T.D. & Tapscott, S.J. Trinucleotide repeat expansion at the myotonic dystrophy locus reduces expression of DMAHP. Nature Genet. 16, 402–406 (1997).
Thornton, C.A., Wymer, J.P., Simmons, Z., McClain, C. & Moxley, R.T. III Expansion of the myotonic dystrophy CTG repeat reduces expression of the flanking DMAHP gene. Nature Genet. 16, 407–409 (1997).
Boucher, C.A. et al. A novel homeodomain-encoding gene is associated with a large CpG island interrupted by the myotonic dystrophy unstable (CTG)n repeat. Hum. Mol. Genet. 4, 1919–1925 (1995).
Heath, S.K., Carne, S., Hoyle, C., Johnson, K.J. & Wells, D.J. Characterisation of expression of mDMAHP, a homeodomain-encoding gene at the murine DM locus. Hum. Mol. Genet. 6, 651–657 (1997).
Behrens, M.I., Jalil, P., Serani, A., Vergara, F. & Alvarez, O. Possible role of apamin-sensitive K+ channels in myotonic dystrophy. Muscle Nerve 17, 1264–1270 (1994).
Ramirez, B.U., Behrens, M.I. & Vergara, C. Neural control of the expression of a Ca(2+)-activated K+ channel involved in the induction of myotonic-like characteristics. Cell. Mol. Neurobiol. 16, 39–49 (1996).
Kawakami, K., Ohto, H., Ikeda, K. & Roeder, R.G. Structure, function and expression of a murine homeobox protein AREC3, a homologue of Drosophila sine oculis gene product, and implication in development. Nucleic Acids Res. 24, 303–310 (1996).
Kawakami, K., Ohto, H., Takizawa, T. & Saito, T. Identification and expression of six family genes in mouse retina. FEBS Lett. 393, 259–263 (1996).
Damiani, E. et al. Skeletal muscle sarcoplasmic reticulum phenotype in myotonic dystrophy. Neuromuscul. Disord. 6, 33–47 (1996).
Cheyette, B.N. et al. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12, 977–996 (1994).
Loosli, F., Winkler, S. & Wittbrodt, J. Six3 overexpression initiates the formation of ectopic retina. Genes Dev. 13, 649–654 (1999).
Pignoni, F. et al. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91, 881–891 (1997).
Ohto, H. et al. Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell. Biol. 19, 6815–6824 (1999).
Winchester, C.L., Ferrier, R.K., Sermoni, A., Clark, B.J. & Johnson, K.J. Characterization of the expression of DMPK and SIX5 in the human eye and implications for pathogenesis in myotonic dystrophy. Hum. Mol. Genet. 8, 481–492 (1999).
Philips, A.V., Timchenko, L.T. & Cooper, T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280, 737–741 (1998).
Amack, J.D., Paguio, A.P. & Mahadevan, M.S. Cis and trans effects of the myotonic dystrophy (DM) mutation in a cell culture model. Hum. Mol. Genet. 8, 1975–1984 (1999).
Timchenko, L.T. Myotonic dystrophy: the role of RNA CUG triplet repeats. Am. J. Hum. Genet. 64, 360–364 (1999).
Harris, S., Moncrieff, C. & Johnson, K. Myotonic dystrophy: will the real gene please step forward. Hum. Mol. Genet. 5, 1417–1423 (1996).
Groenen, P. & Wieringa, B. Expanding the complexity in myotonic dystrophy. Bioessays 20, 901–912 (1998).
Korade-Mirnics, Z., Babitzke, P. & Hoffman, E. Myotonic dystrophy: molecular windows on a complex etiology. Nucleic Acids Res. 26, 1363–1368 (1998).
Ranum, L.P., Rasmussen, P.F., Benzow, K.A., Koob, M.D. & Day, J.W. Genetic mapping of a second myotonic dystrophy locus. Nature Genet. 19, 196–198 (1998).
Day, J.W. et al. Clinical and genetic characteristics of a five-generation family with a novel form of myotonic dystrophy (DM2). Neuromuscul. Disord. 9, 19–27 (1999).
Hiraoka, T. & Clark, J.I. Inhibition of lens opacification during the early stages of cataract formation. Invest. Opthalmol. Vis. Sci. 36, 2550–2555 (1995).
Clark, J.I., Livesey, J.C. & Steele, J.E. Delay or inhibition of rat lens opacification using pantethine and WR-77913. Exp. Eye Res. 62, 75–84 (1996).
Acknowledgements
We thank H. Sarnat for review of the skeletal muscle histology; M. Tallquist for valued advice; and C. Ganders and J. Ilano for technical assistance. This work was supported by NIAMS AR45203 to S.J.T., NEI EY04542 to J.I.C, HD 24875 and HD 25326 to P.S., and a Poncin Scholarship to T.R.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klesert, T., Cho, D., Clark, J. et al. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet 25, 105–109 (2000). https://doi.org/10.1038/75490
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/75490
This article is cited by
-
Polypoidal choroidal vasculopathy in a patient with DMPK-associated myotonic dystrophy
Documenta Ophthalmologica (2022)
-
Dosage effect of multiple genes accounts for multisystem disorder of myotonic dystrophy type 1
Cell Research (2020)
-
Human eye conditions: insights from the fly eye
Human Genetics (2019)
-
Muscleblind-like 3 deficit results in a spectrum of age-associated pathologies observed in myotonic dystrophy
Scientific Reports (2016)
-
Epigenetic mechanisms and genome stability
Clinical Epigenetics (2011)