Abstract
Aim:
To investigate the pharmacodynamics and pharmacokinetics of gemcitabine (dFdC) administered on d 1 and 5 plus cisplatin administered on d 1 in chemonaive patients with stage IIIB or IV non-small cell lung cancer (NSCLC).
Methods:
In each combination cycle, gemcitabine was administered at a dose of 1250 mg/m2 as a 30 min intravenous (iv) infusion on d 1 and 5 followed by cisplatin at a dose of 75 mg/m2 as a 3 h iv infusion on d 1 every 3 weeks. There was an interval of 1 h between the two infusions. Clinical response and toxicity of the regimen were observed. Furthermore, the plasma concentrations of gemcitabine (dFdC) and its metabolite (dFdU) at different time points were detected during the first cycle of infusion. Pharmacokinetic software (PKS) was used to estimate the pharmacokinetic parameters of gemcitabine and its metabolite dFdU.
Results:
A total of 28 patients was enrolled in the study. The median age was 54 years (range 27–75 years), and most patients were in good clinical condition. Twenty-seven patients received two or more treatment cycles. The overall clinical response rate was 33.3%. The median overall survival time was 13 months. The estimated median time to tumor progression (TTP) was 6.2 months, and the 1-year survival rate was 55.6%. Toxicities were tolerated. The main toxicity was myelosuppression; 35.7% of patients had grade 3/4 hematologic toxicities and 28.6% had grade 3/4 non-hematologic toxicities, which were commonly gastrointestinal responses. The pharmacokinetic parameters of dFdC and dFdU were not different between pre- and post-administration of gemcitabine on d 1 and 5. dFdU was minimal (0.729±0.637 μg/mL) before gemcitabine was infused on d 5, and gemcitabine was not present.
Conclusion:
The regimen is active and well tolerated in chemonaive patients with advanced NSCLC. After gemcitabine was administered on d 1 and 5, the pharmacokinetic parameters of dFdC and dFdU showed no difference from those before the infusion, and dFdU was minimal before gemcitabine was administered on d 5.
Similar content being viewed by others
Introduction
Combination chemotherapy with gemcitabine and cisplatin is a well-established therapy for several solid malignancies, including non-small cell lung cancer (NSCLC), pancreatic carcinoma, ovarian cancer and head and neck squamous cell carcinoma, because the two drugs have complementary and synergistic mechanisms of action1, 2. Recently, this regimen has been used extensively in advanced NSCLC3, 4. The dosage and administration of gemcitabine plus cisplatin have been evaluated in several clinical studies (Table 1). Early studies with gemcitabine and cisplatin used a 4-week cycle with gemcitabine given on d 1, 8, and 155, 6, 7, 8. While favorable response rates were observed, the dose schedule also resulted in increased rates of thrombocytopenia and neutropenia. Hematologic toxicity was less severe with a modified regimen employing a 3-week cycle with gemcitabine administration on d 1 and 89, 10, 11, 12, 13. In addition, both gemcitabine and cisplatin were given to patients with stage IIIB or IV NSCLC on the first day of every 2-week period14. However, the optimal schedule of combination chemotherapy with gemcitabine and cisplatin remains unknown.
Currently, a 3-week regimen is commonly and widely used for combination therapy using gemcitabine and cisplatin based on their favorable tolerability profile and clinical benefits with different malignancies. Considering that gemcitabine has low-level toxicity and a short half-life in plasma15, 16, 17, we conducted a modified 3-week regimen (gemcitabine 1250 mg/m2, d 1 and 5 and cisplatin 75 mg/m2, d 1, repeated every 3 weeks) to improve the efficacy and patients' compliance as well as offering a longer chemotherapeutic interval, which is beneficial for the next treatment cycle. To identify the feasibility of this regimen, we carried out a single center phase II clinical trial on the chemonaive patients with advanced NSCLC. First, we evaluated the recent response of patients, including overall response, complete response (CR), partial response (PR) and survival condition (disease progression over time and one-year survival rate). Second, we observed toxicities due to the modified regimen. Finally, we compared the pharmacokinetic parameters of gemcitabine and its metabolite after gemcitabine was administered on d 1 and 5, and we analyzed the residual concentrations of plasma gemcitabine and its metabolite before gemcitabine was administered on d 5.
Materials and methods
Patient eligibility criteria
Patients were eligible if they had histologically or cytologically confirmed stage IIIB or IV NSCLC with an Eastern Cooperative Oncology Group (ECOG) performance status of 0−2 and were chemotherapy-naive, including patients with postoperative recurrence. Other eligibility criteria were as follows: age >18 years; acceptable hematologic parameters [white blood (cell) count (WBC) ≥3.5×109/L, absolute neutrophil count (ANC) ≥1.5×109/L, hemoglobin (HB) ≥10.0 g/L and platelets ≥100×109/L]; and adequate hepatic and renal functions [liver:bilirubin ≤1.5 times the upper limit of normal (ULN), aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ≤1.5 times the ULN or ≤5 times the ULN if hepatic metastases were present; renal:serum creatinine ≤1.5 times the ULN or creatinine clearance ≥50 mL/min]. Patients were excluded for the following reasons: they were pregnant or lactating women; they had serious infection or organic disease; they had central nervous system (CNS) metastasis; they had other malignant tumors except for carcinoma of the cervix uteri in situ and basal cell carcinoma of the skin; or they had other symptoms that could influence the trial. The study was approved by the Ethics Committee of Zhejiang Cancer Hospital, and written informed consent was obtained from all patients.
Treatment plan
In each combination cycle, gemcitabine (Gemzar; 2′,2′-difluoro-2′-deoxycytidine; Eli Lilly & Co, Indianapolis, IN, USA) was administered at a dose of 1250 mg/m2 as a 30 min intravenous (iv) infusion on d 1 and 5 followed by cisplatin [Liaikang; cis-dichloro-platinum; Gejiu Biological and Pharmaceutical Limited Co (or, Gejiu & Co), Yunnan, China] at a dose of 75 mg/m2 as a 3 h iv infusion on d 1 every 3 weeks. There was an interval of 1 h between the two infusions. Treatment was continued until disease progression or for a maximum of six cycles.
Dose modifications were based on weekly blood counts and non-hematologic toxicities. Prior to each cycle, if grade ≥3 non-hematologic toxicities, ANC <1.5×109/L or platelets <100×109/L were present, the treatment was delayed. If these parameters did not recover after 14 d, the patient was removed from the trial. On d 1 of the next cycle, gemcitabine was reduced by 25% and/or cisplatin was reduced by 10% for a grade 4 neutropenic fever, grade 4 neutropenia or thrombocytopenia grade ≥2 with bleeding or platelets <25×109/L occurring during the pre-cycle treatment. If ANC decreased to 0.5×109–0.99×109 or platelets decreased to 50×109–99×109/L on d 5 of each cycle before infusion, the dose of gemcitabine was reduced by 25%. Patients were excluded from the study if they required a third dose reduction or had a non-hematologic toxicity of >3 grade (except for nausea, fatigue or reversible elevation of transaminases).
During the treatment, leukocyte-elevating drugs, such as granulocyte colony stimulating factors, were prohibited during the first cycle and permitted in the following cycles.
Patient evaluation
Prior to chemotherapy, patients underwent a history and physical examination, chest and abdominal computed tomography (CT) scans, complete blood count (CBC), serum biochemistry, urinalysis, and electrocardiogram (ECG). In addition, single photoemission computed tomography (SPECT) and magnetic resonance imaging (MRI) were performed if clinically indicated. A physical examination recording of toxicities and serum biochemistry was performed prior to each cycle of therapy. Biweekly CBC was obtained during each cycle, and CT scans were performed every two cycles.
Response and toxicity analysis
Response to therapy was assessed every two cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST)18, including complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and CR plus PR as the overall response rate (ORR). Treatment was continued until disease progression or for a maximum of six cycles. Toxicity was evaluated every cycle according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
Statistical analysis
Calculations of efficacy parameters were performed using SPSS (version 15.0). Survival and the time from the d of entry until disease progression or the final contact (TTP) were analyzed using Kaplan-Meier estimates. The 95% confidence interval (CI) for tumor response was calculated on the basis of exact binomial statistics. Survival time was calculated from the entry day to the day of death or the last follow up.
Blood sample collection
Blood samples were collected during the first cycle. Approximately 2.5 mL of whole blood was collected from the forearm veins of the patients into heparinized, polypropylene centrifuge tubes before gemcitabine was infused and at 0.25, 0.5, 1, 1.5, 2, 4, 8, 16, 24, 48, and 96 h after infusion. The blood samples were immediately placed on ice. Plasma was obtained by centrifugation at 4000 round per min for 5 min at 4 °C and stored at −20 °C until it was used.
Gemcitabine and dFdU analysis
The concentrations of gemcitabine and dFdU were analyzed as previously described17, 19. Briefly, 1.0 mL of plasma spiked with floxuridine as an internal standard was extracted with 3.0 mL of methanol-acetonitrile (v/v, 1:9). The supernatant was evaporated at 60 °C, and the residue was reconstituted with 0.5 mL of the solution used as the mobile phase. After centrifugation, 50 μL of the supernatant was injected into the HPLC system [Agilent 1100, equipped with a G1311A pump, a G1314A programmable diode array detector (DAD), and a G1313A auto-injector]. Separation was achieved on a Lichrospher C18 (4.6 mm×250 mm, 5 μm) column at 25 °C with the flow rate of the mobile phase set to 0.8 mL/min. The compounds were detected at 268 nm. The mobile phase consisted of 40.0 mmol/L acetate ammonium buffer solution (pH 5.5) and acetonitrile (v/v, 97.5:2.5). The linear range was 0.20−10.0 μg/mL (r=0.9999) for dFdC and 0.50−50.0 μg/mL (r=0.9999) for dFdU. The limit of detection (LOD) was 0.10 μg/mL for dFdC and 0.25 μg/mL for dFdU, whereas the limit of quantification (LOQ) was 0.20 μg/mL (RSD<10%) for dFdC and 0.50 μg/mL (RSD<3%) for dFdU. The average recovery of dFdC and dFdU was 103.3% and 98.7%, respectively. For intra- and inter-day measurements, the corresponding standard deviations of the measurements of dFdC and dFdU were both less than 5.5%.
Pharmacokinetic studies and analysis
After the concentrations of gemcitabine and dFdU were analyzed, gemcitabine and dFdU plasma concentration data were obtained at different time periods. PKS analysis (DAS, Drug and Statistics version 2.1.1, Mathematical Pharmacology Professional Committee of China, Shanghai, China) was used to estimate the following pharmacokinetic parameters: area under the concentration versus time curve (AUC), elimination half-life (t1/2), total body clearance (CL) and volume of distribution (Vd). To determine whether gemcitabine and dFdU pharmacokinetic parameters were altered on d 1 and 5, a paired-samples t-test was used to compare the pharmacokinetic parameters between d 1 and 5, and P-values <0.05 were considered statistically significant.
Results
Patient characteristics
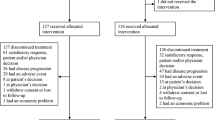
Twenty-eight patients (15 males and 13 females) with NSCLC were enrolled in the study between October 2006 and October 2007. The patient characteristics are described in Table 2. The median age was 54 years (range 27–75 years), and 22 patients had an ECOG performance status of 0 or 1. All patients were in an advanced stage and were chemotherapy-naive, including one patient with postoperative recurrence. Twenty-one patients had adenocarcinoma, four had squamous carcinoma and one patient had large cell carcinoma.
Treatment received
A total of 104 cycles of chemotherapy was completed with a median number of four cycles (range one to six); 27 patients received two or more treatment cycles, and the remaining patient was excluded due to grade 4 gastrointestinal (GI) toxicity before the second treatment cycle. On d 1 of all cycles, 10 patients (36%) postponed the administration and their dosages were reduced. Among these patients, the gemcitabine dose was reduced for five (18%), and the cisplatin dose was reduced for three patients (11%). On d 5 of all cycles, the dose of gemcitabine was reduced for six patients (21%). The most common reasons for dose reduction were neutropenia and thrombocytopenia. The average hospitalization time was 7 d.
Efficacy
There was no CR case in the 27 evaluated patients. Nine patients (33.3%; 95% CI, 16.52% to 53.96%) showed a PR; therefore, the overall clinical response rate was 33.3%. Fifteen patients (55.6%; 95% CI, 35.33% to 74.52%) had stable disease, three (11.1%; 95% CI, 2.35% to 29.16%) progressed and the disease stable rate (CR+PR+SD) was 88.9% (95% CI, 70.84% to 97.65%). For the 27 patients, the median overall survival time was 13 months (95% CI, 9.0 to 15.0), and the median TTP and one-year survival rate was 6.2 months (95% CI, 4.5 to 6.8) and 55.6% (95% CI, 36.86% to 74.34%), respectively.
Toxicity
Toxicities were evaluated in all 28 patients. The main toxicities detected were hematologic toxicities, including anemia (grade 3/4, six cases), neutropenia (grade 3/4, eleven cases) and thrombocytopenia (grade 3/4, seven cases). Eight patients had febrile neutropenia (grade 3/4, one case), three had infection (grade 3/4, one case) and eight had grade 3/4 non-hematologic toxicities, which were commonly GI responses. Five had rashes (grade 1/2), seven had peripheral neuropathy toxicity (grade 1/2) and two had increased urea nitrogen (grade 1). No deaths were induced by the treatment. Grade 3/4 toxicities are listed in Table 3.
Gemcitabine and dFdU analysis
The plasma concentrations of gemcitabine and its metabolite dFdU were analyzed by HPLC in seven patients at different time points before or after gemcitabine administration (Figure 1A and Figure 1B). The results showed that there was no residual dFdC and minimal residual dFdU (0.729±0.637 μg/mL) before gemcitabine was infused on d 5.
Pharmacokinetic analysis
Blood concentration time data of gemcitabine and dFdU were calculated using PKS. The results for both gemcitabine and its metabolite (dFdU) fitted biphasic kinetic models, and their pharmacokinetic parameters are presented in Table 4. The plasma pharmacokinetic parameters CL, Vd, AUC, t1/2, and Cmax of gemcitabine and dFdU were similar after gemcitabine was administered on d 1 and 5, and there were no statistically significant differences (paired-samples t-test; P>0.05).
Discussion
Disagreements regarding the optimal schedule of administration for gemcitabine in combination with cisplatin are still common. Currently, a 3-week regimen is typically accepted. In most of the 3-week regimen trials, gemcitabine was usually administered on d 1 and 8. In this study, we used modified 3-week regimen in which gemcitabine (1250 mg/m2 on d 1 and 5) and cisplatin (75 mg/m2 on d 1) intravenously within one week, and treatment was repeated every 3 weeks. We investigated the pharmacodynamics and pharmacokinetics in 28 chemonaive patients with advanced NSCLC. The results reveal that the therapeutic effect is favorable. The partial response rate was 33.3%, and the disease stability rate was 88.9%. The median overall survival time was 13 months, and the median TTP and one-year survival rate was 6.2 months and 55.6%, respectively. These results are similar to those reported in Table 19, 11. The main toxicity was myelosuppression. Ten patients (35.7%) had grade 3/4 hematologic toxicities, consisting of three infected cases (grade 3/4, one case) and eight febrile neutropenias (grade 3/4, one case). Eight patients (28.6%) had grade 3/4 non-hematologic toxicities, which were commonly GI responses. No patients died during the treatment. The majority of the dose modifications occurred within the first and second treatment cycles. During the treatment cycles, the incidence of grade 3 neutropenia was gradually lower, indicating that the dose modifications could effectively regulate the risk of severe neutropenia.
Gemcitabine (dFdC) is a prodrug that, after intracellular phosphorylation, exerts its cytotoxic effects through its active intracellular metabolites: gemcitabine di-phosphate and tri-phosphate. After administration, dFdC is rapidly metabolized by deamination in the liver, kidney and other tissues to a noncytotoxic metabolite (2,2′-difluorodeoxy-uridine, dFdU)17. Gemcitabine is known to have a half-life of 11–30 min following a 30-min infusion, while dFdU has a long half-life that varies from 8 h to 84 h15, 16, 17, 20, 21. Although dFdU is the inactive metabolite, it has been reported that longer durations of exposure to dFdU can influence the metabolic process of gemcitabine and significantly increase the cytotoxicity of dFdC22, 23. Therefore, the plasma concentrations of gemcitabine and its metabolite (dFdU) at different time points need to be detected during the first cycle of administration.
Accumulation of gemcitabine and its metabolite is therefore not likely to be measurable based on the dosing interval of 96 h (from d 1 to d 5). Our data show that the maximum plasma concentration of gemcitabine was observed at the end of the infusion, and it was undetectable in plasma before infusion on d 5. Moreover, the plasma pharmacokinetic parameters CL, Vd, AUC, t1/2, and Cmax were similar after its administration on d 1 and 5, and no significant differences were identified (paired-samples t-test; P>0.05). As for the gemcitabine metabolite dFdU, four of the seven patients had minimal residual dFdU in the plasma before administration on d 5 (0.729±0.637 μg/mL). However, the plasma pharmacokinetic parameters CL, Vd, AUC, t1/2, and Cmax of dFdU were also similar after gemcitabine was administered on d 1 and 5, and no significant differences were found (paired-samples t-test between d 1 and 5; P>0.05). This suggests that the residual dFdU before administration on d 5 had no influence on the physiological disposition of gemcitabine and its metabolite after its administration. Based on these data, it is clear that there was no cumulative effect of gemcitabine or its metabolite when adopting the modified 3-week regimen, which is consistent with the results previously reported15. In a previous study, gemcitabine was administered over two courses, with each course consisting of a 30-min infusion at 1000 mg/m2 per week for 3 weeks followed by 1 week of rest. No gemcitabine was detectable before starting the next infusion in either course 1 or 2. In several patients, 1–6 μmol/L dFdU was detectable before starting the next infusion on d 8 or 15 during each course. This residual concentration of dFdU on d 8 and 15 did not result in a higher accumulation ratio (R) on either day compared to d 1 in either of the two courses. The authors concluded that gemcitabine can be administered safely without the risk of drug accumulation. According to these data, the toxicities induced by gemcitabine have almost no relationship with the accumulation of its metabolite.
Besides dosage and administration, the drug metabolic process in vivo also correlates with the drug combination, the blood sampling time and patient characteristics such as race, age and gender. In this research, the elimination of gemcitabine and its metabolite were fitted to the two-compartment model according to their concentration time curves. While the main pharmacokinetic parameters were comparable to previous reports24, 25, the AUC of dFdU estimated from our study was increased. Additionally, the CL of dFdU was lower than those studies. Thus, further research is needed.
In summary, the current study demonstrates that the modified 3-week regimen is effective and well tolerated in chemonaive patients with stage IIIB or IV NSCLC. After gemcitabine was administered at a dose of 1250 mg/m2 as a 30 min iv infusion on d 1 and 5 followed by cisplatin at a dose of 75 mg/m2 as a 3 h iv infusion on d 1, there was minimal residual dFdU in the plasma before administration on d 5; however, there was no difference between the pharmacokinetic parameters of dFdC and dFdU. Compared to the standard 3-week regimen (gemcitabine administered on d 1 and 8), this regimen could improve patients' compliance as well as offering a longer chemotherapeutic interval, which is beneficial for the next treatment cycle. Although no evidence of better clinical responses was observed, the data from this study with the modified 3-week regimen on the therapeutic effect and toxicity are still very encouraging. Further randomized controlled studies versus the standard 3-week regimen are wanted before new guidelines can be proposed.
Author contribution
Neng-ming LIN and Sheng-lin MA designed the research; Yun FAN, Lü-hong LUO, Luo FANG, Zhi-yu HUANG, Hai-feng YU and Feng-qin WU performed the research; Yun FAN and Neng-ming LIN analyzed the data; Neng-ming LIN wrote the paper.
References
Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ . Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 1996; 2: 521–30.
Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ . Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol 1995; 22 (4 Suppl 11): 72–9.
Belani CP . Chemotherapy regimens in advanced non-small-cell lung cancer: recent randomized trials. Clin Lung Cancer 2000; 2: S7–S10.
Le Chevalier T, Scagliotti G, Natale R, Danson S, Rosell R, Stahel R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer 2005; 47: 69–80.
Rossi D, Graziano F, Catalano V, Giordani P, Fedeli SL, Alessandroni P, et al. A new cisplatin/gemcitabine schedule in locally advanced (IIIB) and metastatic (IV) non-small cell lung cancer: relationship between dose-intensity and efficacy. A phase II study. Anticancer Res 2002; 22: 3087–92.
Jassem J, Krzakowski M, Roszkowski K, Ramlau R, Słomiński JM, Szczesna A, et al. A phase II study of gemcitabine plus cisplatin in patients with advanced non-small cell lung cancer: clinical outcomes and quality of life. Lung Cancer 2002; 35: 73–9.
van Zandwijk N, Smit EF, Kramer GW, Schramel F, Gans S, Festen J, et al. Gemcitabine and cisplatin as induction regimen for patients with biopsy-proven stage IIIA N2 non-small-cell lung cancer: a phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group (EORTC 08955). J Clin Oncol 2000; 18: 2658–64.
Shepherd FA, Cormier Y, Burkes R, Evans WK, Goss G, Klimo P, et al. Phase II trial of gemcitabine and weekly cisplatin for advanced non-small cell lung cancer. Semin Oncol 1997; 24: S8-27–S8-30.
Akcali Z, Calikusu Z, Sakalli H, Ozyilkan O . Gemcitabine and cisplatin treatment of advanced-stage non-small-cell lung cancer in patients given cisplatin on day 8. Tumori 2008; 94: 474–80.
Aydiner A, Kiyik M, Cikrikcioglu S, Kosar F, Gurses A, Turna A, et al. Gemcitabine and cisplatin as neo-adjuvant chemotherapy for non-small cell lung cancer: a phase II study. Lung Cancer 2007; 58: 246–52.
Aydiner A, Kiyik M, Cikrikcioglu S, Kosar F, Gurses A, Turna A, et al. A phase II study with gemcitabine and split-dose cisplatin in patients with advanced non-small cell lung cancer. Lung Cancer 2006; 54: 57–62.
Parra HS, Cavina R, Latteri F, Campagnoli E, Morenghi E, Torri W, et al. Cisplatin plus gemcitabine on days 1 and 4 every 21 days for solid tumors: result of a dose-intensity study. Invest New Drugs 2006; 25: 57–62.
Zwitter M, Kovac V, Smrdel U, Kocijancic I, Segedin B, Vrankar M . Phase I–II trial of low-dose gemcitabine in prolonged infusion and cisplatin for advanced non-small cell lung cancer. Anticancer Drugs 2005; 16: 1129–34.
López-Vivanco G, Viteri A, Barceló R, Muñoz A, Rubio I, Mañé JM, et al. Biweekly administration of cisplatin/gemcitabine in advanced non small cell lung cancer. Am J Clin Oncol 2005; 28: 501–7.
de Lange SM, van der Born K, Kroep JR, Jensen HA, Pfeiffer P, Cleverly A, et al. No evidence of gemcitabine accumulation during weekly administration. Eur J Clin Pharmacol 2005; 61: 843–9.
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 1991; 9: 491–8.
Lin NM, Zeng S, Ma SL, Fan Y, Zhong HJ, Fang L . Determination of gemcitabine and its metabolite in human plasma using high-pressure liquid chromatography coupled with a diode array detector. Acta Pharmacol Sin 2004; 25: 1584–9.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–16.
Lin NM, Zeng S, Ma SL, Fan Y, Zhong HJ, Fang L . Pharmacokinetics study of gemcitabine and its metabolite in Chinese patients with malignant tumor. Chin Pharm J 2005; 40: 1089–92.
Hui YF, Reitz J . Gemcitabine: a cytidine analogue active against solid tumors. Am J Health Syst Pharm 1997; 54: 162–70.
Storniolo AM, Allerheiligen SR, Pearce HL . Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol 1997; 24: S7-2–S7-7.
Stephan AV, Dick P, Maria AJ, Bolijn MJ, Ong FH, Govindarajan R, et al. New insights into the pharmacology and cytotoxicity of gemcitabine and 2,2-difluorodeoxyuridine. Mol cancer Ther 2008; 7: 2415–25.
Veltkamp SA, Pluim D, van Tellingen O, Beijnen JH, Schellens JH . Extensive metabolism and hepatic accumulation of gemcitabine after multiple oral and intravenous administration in mice. Drug Metab Dispo 2008; 36: 1606–15.
Kuenen BC, Rosen L, Smit EF, Parson MR, Levi M, Ruijter R, et al. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol 2002; 20: 1657–67.
Gietema JA, Hoekstra R, de Vos FY, Uges DR, van der Gaast A, Groen HJ, et al. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann Oncol 2006; 17: 1320–7.
Acknowledgements
This study was financially supported by a grant from the Medical Science Research Foundation of Zhejiang Province, China (No 2008B027). We would like to thank Eli Lilly Company for providing a sample of dFdC.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fan, Y., Lin, Nm., Ma, Sl. et al. Phase II trial of gemcitabine plus cisplatin in patients with advanced non-small cell lung cancer. Acta Pharmacol Sin 31, 746–752 (2010). https://doi.org/10.1038/aps.2010.50
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.50
Keywords
This article is cited by
-
Experience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma
European Archives of Oto-Rhino-Laryngology (2012)