Abstract
Heat shock protein 90 (Hsp90) is a promising target for anti-tumor therapy. We previously reported the anti-tumor activity of a novel Hsp90 inhibitor, KW-2478, in multiple myeloma (MM) as a single agent. In this study, we examined the combinational effect of KW-2478 and bortezomib, a proteasome inhibitor, in vitro and in vivo. In vitro, KW-2478 enhanced bortezomib-induced cell growth inhibition, both in MM cell lines and primary patient MM cells. The combination of KW-2478 and bortezomib also induced caspase activation in MM cell lines. Interestingly, the combination synergistically enhanced the expression of Hsp70B, a homolog of Hsp70, in human MM cells and peripheral blood mononuclear cells, indicating Hsp70B could be a surrogate biomarker for the combination of Hsp90 and proteasome inhibitors. In vivo, the combination of KW-2478 with bortezomib showed synergistic anti-tumor activity without significant body weight loss in a subcutaneously inoculated human myeloma model. Furthermore, the combination also showed synergistic reduction of tumor burden in bone marrow in an orthotopic myeloma model. Our results strongly suggest that combination of KW-2478 with bortezomib could exhibit enhanced anti-tumor activity against human myeloma.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a plasma-cell malignancy characterized by accumulation of malignant cells in the bone marrow and production of a monoclonal immunoglobulin (M protein). Treatment strategies for MM have changed substantially over the past 10 years following the introduction of bortezomib and the immunomodulatory drugs, thalidomide and lenalidomide. Although these drugs have improved patient survival, the disease is still incurable in the majority of patients. There is therefore an unmet need for novel treatment strategies.1, 2, 3, 4
Bortezomib, a first-in-class proteasome inhibitor, exerts anti-MM effects through multiple mechanisms,5 including apoptosis induction though ER stress. As MM cells constantly produce a large amount of M protein, they are susceptible to ER stress; inhibition of the proteasome results in accumulation of immunoglobulin-derived defective ribosomal products, namely misfolded/unfolded proteins (UPR), and this accumulation causes apoptosis following extensive ER stress.6 The activity of bortezomib may therefore be enhanced by combination with drugs that induce misfolded/UPR accumulation.
Heat shock protein 90 (Hsp90) is a ubiquitously expressed molecular chaperone, that facilitates the folding and stability of numerous signaling molecules that control the growth and survival of cancer cells.7 Hsp90 also has a crucial role in chaperoning immunoglobulins, including M protein. The interruption of this chaperoning activity by the inhibition of Hsp90 leads to accumulation of misfolded/unfolded proteins.8 On the basis of the above scenario, Hsp90 inhibitors could be an ideal drug to enhance the anti-myeloma activity of bortezomib. Several preclinical studies have already reported synergistic anti-myeloma activity with combinations of Hsp90 inhibitors and bortezomib.9, 10, 11, 12 Furthermore, tanespimycin (17-AAG), a first generation Hsp90 inhibitor, with some limitations such as low water solubility and hepatotoxicity, has demonstrated promising activity against relapsed/refractory MM when combined with bortezomib in clinical trials.13
KW-2478 is a second generation Hsp90 inhibitor of non-natural product that overcomes the limitations of tanespimycin described above. Recently, we have reported the anti-tumor activity of KW-2478 in MM cells as a single agent.14 The purpose of the present study is to investigate the anti-myeloma activity of the combination of KW-2478 and bortezomib in vitro and in vivo preclinical models.
Materials and methods
Reagents
KW-2478 was synthesized at Kyowa Hakko Kirin Co., Ltd. Bortezomib was synthesized at Kyowa Hakko Kirin Co., Ltd or purchased from Millennium Pharmaceuticals, Inc. (Cambridge, MA, USA). For in vitro use, both compounds were dissolved in dimethyl sulfoxide and diluted in culture medium. For in vivo use, both compounds were dissolved in saline.
MM-derived cell lines
OPM-2/GFP (green fluorescent protein) cell line, OPM-2 cell line 15 stably transfected with GFP gene, was obtained from Anticancer (San Diego, CA, USA) and maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 vol% heat-inactivated fetal bovine serum (Invitrogen) and 1 vol% penicillin–streptomycin solution (Invitrogen).
NCI-H929 cell line16 was obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640 medium supplemented with 10 vol% fetal bovine serum, 1 mmol/l sodium pyruvate (Invitrogen), 10 mmol/l HEPES (Invitrogen), 4.5 g/l D-glucose (Sigma-Aldrich, St Louis, MO, USA) and 50 μmol/l 2-mercaptoethanol (Invitrogen).
Measurement of cell viability and caspase-3 like activity of cell lines
OPM-2/GFP cells were plated into 96-well V-bottom plates and treated with KW-2478, bortezomib or their combinations. After 3 h, the cells were washed with culture medium twice, and further incubated in drug-free medium for 21 h to measure apoptosis induction or 45 h to measure cell viability. For measurement of cell viability, Cell Proliferation Reagent WST-8 reagent (Cell Counting Kit-8, Dojindo Molecular Technologies, Kumamoto, Japan) was used.
For measurement of apoptosis induction, caspase-3 like protease (DEVDase) activity was measured using the fluorogenic substrate acetyl-L-aspartyl-L-glutamyl-L-valyl-L-aspartic acid-7-amino-4-methylcoumarin (DEVD-AMC, Peptide Institute, Osaka, Japan), as described previously.17
Determination of synergism by the combination index (CI) method
The interaction of KW-2478 and bortezomib was determined by CI methods using the CompuSyn software program (ComboSyn Inc., Paramus, NJ, USA) to determine whether the combination was additive or synergistic. Data from cell viability assays were expressed as the fraction of antiproliferative activity (cell viability loss) by the individual drugs or the combination in drug-treated cells, and CI values between two drugs were generated. A CI of 1 indicates an additive effect, whereas a CI of <1 indicates synergy. A CI value at 50% effect (Fa=0.5) based on the CI isobologram equation was also calculated.18
Measurement of drug activity in primary MM cells
Bone marrow aspirates were obtained at relapse from MM patients following written informed consent and with the approval of the local research ethics committee. MM cells were enriched for the plasma-cell fraction by negative selection using the RosetteSep human MM cell enrichment cocktail (StemCell Technologies, Grenoble, France) and Ficoll-Hypaque (Nycomed, Oslo, Norway) density-gradient centrifugation. Separated MM cells collected from the plasma/Ficoll interface were washed in PBS and cryopreserved in 50% FCS, 40% RPMI, 10% dimethyl sulfoxide in liquid nitrogen for subsequent use.
The activity of KW-2478 and bortezomib alone, and in combination, was studied in 10 primary MM samples using HS-5 cells (ATCC) as the stromal component in a co-culture system.19 HS-5 cells (2.5 × 104 cells/ml) in serum-free DMEM (ATCC) were allowed to adhere overnight at 37 °C before the addition of primary MM cells at a concentration of 1 × 105 cells/ml in standard RPMI 1640 medium (Sigma Aldrich Co., Gillingham, Dorset, UK). Plates were incubated at 37 °C for a further 2 h to allow for stromal/MM cell interaction, before the addition of study drugs. After a 48 h incubation 100 μl of cell suspension (containing the MM cells) was removed for the determination of cell viability using the Guava Via Count assay (Guava Technologies, Inc., Hayward, CA, USA).
Detection of apoptosis and UPR-associated proteins
Cultured cells
OPM-2/GFP and NCI-H929 cells were plated into 6-well plates and treated with KW-2478, bortezomib or combinations of both. After 3 h the cells were washed twice with PBS and further incubated in drug-free medium for 21 h. After the total of 24 h incubation, cells were lysed with NP40 cell lysis buffer (Invitrogen) containing 1 mmol/l phenylmethanesulfonyl fluoride and 1 vol% protease inhibitor cocktail (Sigma Aldrich). Proteins from each cell lysate were separated by SDS–PAGE, transferred to polyvinylidene fluoride membranes and immuno-blotted with primary antibodies to Caspase-2 (Cat#551093, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), Caspase-9 (Cat#M054-3, Medical and Biological Laboratories, Nagoya, Japan), Cleaved Caspase-3 (Cat#9661s, Cell Signaling Technology, Danvers, MA, USA), PARP (Cat#4338-MC-50, Travigen, Gaithersburg, MD, USA), Noxa (Cat#sc-56169, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Hsp70 (Cat#SPA-810, Stressgen Bioreagents, Farmingdale, NY, USA), Hsp27 (Cat#2402, Cell Signaling Technology), Grp78 (Cat#610978, Becton, Dickinson and Company), CHOP (Cat#2895s, Cell Signaling Technology) or β-actin (Cat#A5441, Sigma Aldrich). The bands were detected using appropriate secondary antibodies (horseradish peroxidase-linked anti-mouse antibody (GE Healthcare UK Ltd, Buckinghamshire, England) or anti-rabbit antibody (GE Healthcare)) and Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA).
Human peripheral blood mononuclear cells (hPBMCs)
Peripheral bloods were obtained from three healthy volunteers following written informed consent. The hPBMCs were separated by Ficoll-Paque PLUS (GE Healthcare) density centrifugation. Three million hPBMCs suspended in 1 ml of the assay medium (RPMI 1640 medium supplemented with 10 vol% fetal bovine serum and 1 vol% penicillin–streptomycin solution) were plated into 24-well plates, and treated with KW-2478, bortezomib or combinations. After 3 h, the cells were washed twice with PBS, and further incubated in drug-free medium for 3 h. After the total of 6 h incubation period, the cells were lysed with NP40 cell lysis buffer (Invitrogen) containing 1 mmol/l phenylmethanesulfonyl fluoride and 1 vol% protease inhibitor cocktail. Detection of apoptosis related proteins, Hsp70, Hsp70B and Hsp27 in each cell lysate was performed as described above.
Anti-tumor activity in NCI-H929 subcutaneous model
Details of establishing the NCI-H929 s.c. bearing severe combined immunodeficient mice model were described previously.14 Mice bearing s.c. NCI-H929 tumors ranging from 153.74 to 294.03 mm3 were randomly assigned into cohorts of five mice each. The treatments for each group were as follows; (1) No treat (control), (2) bortezomib, 1 mg/kg, biw × 2 (on days 0, 3, 7, 10), i.v., (3) KW-2478, 50 mg/kg, biw × 2 (on day 0, 3, 7, 10), i.v., and (4) KW-2478, 50 mg/kg, biw + bortezomib, 1 mg/kg, biw × 2. Body weight and tumor volume of mice were measured at least twice a week. Tumor volume was calculated by the following formula: Tumor volume=DL × DS × DS × 1/2 (DL, long diameter; DS, short diameter). Relative tumor volume was represented by a V/V0 value (V0, initial tumor volume; V, tumor volume after dosing). The relative V/V0 values on each day were converted to a T/C value (C is V/V0 of control mouse; T is V/V0 of treated mouse).
Fourteen days after the initial administration (on day 14), blood samples were obtained from each mouse followed by measurement of serum M protein (Igκ chain) with Human Kappa ELISA Quantization Kit (Bethyl Laboratories, Montgomery, TX, USA).
Anti-tumor activity in OPM-2/GFP orthotopic model
Details of establishing the OPM-2/GFP orthotopic model were described previously.14 The day when OPM-2/GFP cells were i.v. inoculated was designated as day 0. On day 26, serum M protein (Igλ chain) of each mouse was measured using the Human Lambda ELISA Quantitation Kit (Bethyl Laboratories). Mice with serum M protein concentrations on day 26 ranging from 162 to 523 ng/ml were divided into 6 groups (n=5 in each group) on day 27 so that the mean serum M protein concentrations in each group were similar. The treatments for each group were as follows; (1) Saline (control), (2) KW-2478, 100 mg/kg, biw × 2 (on day 27, 30, 34, 37), i.v., (3) KW-2478, 200 mg/kg, biw × 2 (on day 27, 30, 34, 37), i.v., (4) bortezomib, 0.3 mg/kg, biw × 2 (on Days 27, 30, 34, 37), i.v., (5) KW-2478, 100 mg/kg+bortezomib, 0.3 mg/kg, and (6) KW-2478, 200 mg/kg+bortezomib, 0.3 mg/kg. On day 40, serum M protein concentrations were measured as described above. On day 50, the GFP fluorescence in the tumor bearing mice was measured using the AQUACOSMOS/Macro Fluorescence System (Hamamatsu Photonics, Shizuoka, Japan). To confirm that the fluorescence represented tumor burden, limbs with fluorescence from some animals were formalin fixed, and the paraffin sections were hematoxylin and eosin stained and histochemically examined.
Statistical methods for analysis of raw data
Tumor volume
The interaction between KW-2478 and bortezomib with regard to anti-tumor activity was evaluated using the fractional product method that allows an evaluation of potential synergy at a defined level of effect. In the fractional product method, the effect of two independently acting agents is defined as the product of the unaffected fractions after treatment with either drug alone: fu (1,2)=fu (1) × fu (2).20 This formula allows the predicted effect of co-administration to be calculated on the basis of the assumption that the two agents do not interact or cooperate in inducing their effects. If the T/C value after combined administration with KW-2478 and bortezomib is below the calculated product, T/C combination=T/C KW-2478 × T/C bortezomib, then the two drugs show synergy.
Statistical analysis was performed using the SAS software program (Release 9.1.3, SAS Institute Inc., Cary, NC, USA), in which the tumor volume on each day was used as a parameter. Bartlett's test was used to test if the samples have equal variances. When the variance was equal (P>0.05), analysis of variance among each group was analyzed by one-way analysis of variance test, and subsequently significant differences between selected two groups were analyzed by Tukey test. When the variance was not equal, analysis of variance among each group was analyzed by Kruskal–Wallis test, and subsequently significant differences between selected two groups were analyzed by Steel–Dwass test. In these tests, P<0.05 was considered significant.
Serum M protein
The mean and standard deviation (s.d.) of each group were calculated. Statistical analysis was performed using the SAS software program (Release 9.1.3, SAS Institute Inc.). Analysis of variance among each group was analyzed by one-way analysis of variance test, and subsequently significant differences between selected two groups were analyzed by Tukey’s test. In this test, P<0.05 was considered significant.
Results
Apoptosis induced by bortezomib combined with KW-2478 in MM cell lines
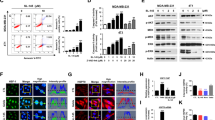
First, we examined cell-killing activity of KW-2478, bortezomib, or the combination of both, against a MM cell line, OPM-2/GFP. OPM-2/GFP cell has an activating mutation in the FGFR3 locus and we have previously reported that KW-2478 cytoxicity in these cells is associated with depletion of FGFR3 with 72 h continuous exposure.14 We are now examining the anti-tumor activity of KW-2478 in combination with bortezomib in a clinical trial in which both agents are administered in an intermittent schedule. In the present study we have therefore adopted a pulsed exposure, with a duration of 3 h, as HSP90 inhibitory concentrations of KW-2478 are achieved for 3 h after dose in MM patients based on data from PK studies (data not shown). As shown in Figure 1a, although KW-2478 alone had little effect on cell viability with 3 h treatment followed by a wash-out period, it potentiated the cell-killing activity of bortezomib in a concentration dependent manner. Using the fraction of antiproliferative activity (Fraction affected, Fa), the combinatorial effect of KW-2478 with bortezomib was evaluated by CI plot at five combination concentrations, with a synergistic effect observed at the three highest concentration. The addition of KW-2478 (at 2.2 to 5 μmol/l) to bortezomib (at 0.022 to 0.05 μmol/l) showed CI values of 0.76 to 0.82, suggesting that this combination was synergistic across a wide range of concentrations (Figure 1b).
Combinatorial effect of KW-2478 (KW) and bortezomib (BTZ) on proliferation and apoptosis of OPM-2/GFP cells. (a) OPM-2/GFP cells were treated with KW, BTZ, or their combinations for 3 h, and further incubated in drug-free medium for 45 h, followed by determination of cell viability. The results are represented as percent of cell viability in drug-treated cells relative to untreated control cells. Each column represents the mean+s.d. of three independent experiments. (b) Combinatorial effect of KW-2478 and bortezomib on CI plot in OPM-2/GFP cells. CI defining the interaction between KW-2478 and botezomib are plotted against the fraction of antiproliferative activity. CI values: 1, additive; <1, synergistic. (c) OPM-2/GFP cells were treated with KW, BTZ alone, or their combinations for 3 h, and further incubated in drug-free medium for 21 h, followed by measuring the cacpase-3 like proteinase (DEVDase) activity. Data represent the mean+s.d. of three independent experiments.
Effect of KW-2478 on apoptosis induction, indicated as DEVDase activity, by bortezomib was also examined in OPM-2/GFP cells. Although KW-2478 alone induced little caspase activation, the addition of 0.44 to 3.3 μmol/l KW-2478 enhanced caspase activation by bortezomib at concentrations ranging from 0.01 to 0.05 μmol/l (Figure 1c). Apoptosis induction by the combination of KW-2478 and bortezomib was confirmed by caspase-2, 3, 9 and PARP cleavage (caspase-2 and -9 represent the uncleaved forms, with reductions in band intensity indicating cleavage (Figure 2a)). Apoptosis induction, indicated as caspase-3, 9 and PARP cleavage, was also seen in NCI-H929 cells (Figure 2b).
Apoptosis and UPR-associated proteins induced by KW-2478 and bortezomib (BTZ) in MM cell lines. (a) OPM-2/GFP cells were treated with KW-2478, (BTZ) alone, or their combinations for 3 h, and further incubated in drug-free medium for 21 h, followed by western blotting. (b) NCI-H929 cells were examined as described in (a).
It has been reported that Noxa, a BH3-only member of the Bcl-2 family, is a mediator of apoptosis after exposure to bortezomib.21, 22, 23 Consistent with these reports, the expression of Noxa was induced by bortezomib in OPM-2/GFP cells (Figure 2a). While KW-2478 alone had little effect on Noxa expression, the addition of KW-2478 to bortezomib resulted in a further increase in Noxa compared with bortezomib alone. This data suggest that KW-2478 enhanced apoptosis by increasing Noxa induction in OPM-2/GFP cells. In NCI-H929 cells Noxa levels remained very low.
UPR-associated proteins induced by KW-2478 and bortezomib in MM cell lines
As it is well established that Hsp90 inhibition results in increased Hsp70 expression, an effect often used as a surrogate marker of Hsp90 inhibition, we examined expression of Hsp70 and Hsp70B, an Hsp70 homolog, in treated cells. As shown in Figure 2, KW-2478 treatment increased the level of Hsp70, as expected, and this effect was enhanced by the addition of bortezomib in both OPM-2/GFP and NCI-H929 cell lines. To our surprise, although we could not detect Hsp70B in NCI-H929 cells, a marked induction in Hsp70B was found only in the combination of KW-2478 and bortezomib in OPM-2/GFP (Figure 2a). This finding indicates that induction of Hsp70B, a secondary responder to proteotoxic stress, could be a surrogate biomarker specific to the combination of KW-2478 and bortezomib, as little or no basal expression was observed in untreated cells.24 As an increase in the expression of the ER-associated chaperone, Grp78, (also known as BiP), has been associated with bortezomib-induced apoptosis in MM cell lines,25, 26 we investigated its induction in both cell lines. No change in Grp78 was observed in OPM-2/GFP cells (Figure 2a). In NCI-H929 cells, bortezomib induced Grp78 at all concentrations, but KW-2478 had little or no effect on Grp78 expression levels (Figure 2b).
Cytotoxicity in MM patient samples
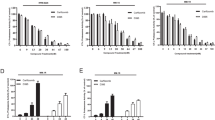
Anti-MM activity of the combination of KW-2478 and bortezomib against 10 different primary MM samples was evaluated in the HS-5 co-culture system (Figure 3). As with the cell line data reported in Figure 1a, minimally toxic concentrations of KW-2478 (1 and 2 μmol/l) increased the activity of bortezomib, an effect that was statistically significant in three of the four combinations studied. It is noteworthy that not all primary samples showed an increased effect with the combination, an observation most apparent in several samples at 3 nmol/l bortezomib.
Hsp70B as a possible surrogate biomarker of KW-2478 and bortezomib combination
To verify if Hsp70B induction might be useful as a surrogate biomarker of the combination therapy, we examined Hsp70B induction using hPBMCs from healthy donors. As shown in Figure 4, bortezomib alone had no effect on Hsp70B at any concentration studied. Although KW-2478 alone induced Hsp70 in all three samples, and Hsp27 in two of the three, KW-2478 alone had only a small effect on Hsp70B in two samples. However, significant induction of Hsp70B was observed when KW-2478 and bortezomib were combined, particularly at higher concentrations of KW-2478. This provides further evidence that Hsp70B may be useful as a surrogate biomarker specific to the combination of bortezomib and KW-2478.
KW-2478 enhanced anti-tumor activity of bortezomib in s.c. xenograft model
We next investigated in vivo anti-tumor activity in an NCI-H929 s.c. xenograft model using a twice-weekly administration schedule for 2 weeks. Although KW-2478 or bortezomib alone showed moderate anti-tumor effects, activity was clearly more marked when the two agents were combined (Figure 5a). The differences between each single agent (KW-2478 or bortezomib) and the combination group were statistically significant on day 14. The observed T/C values in the combination group were smaller than calculated T/C values based on the T/C values for each agent alone, confirming a synergistic interaction (Table 1).
Anti-tumor activity in NCI-H929 s.c. xenograft model. (a) NCI-H929 cells (1 × 107 cells/mouse) were inoculated s.c. into the flank of severe combined immunodeficient mice. After 10 days, KW-2478 (KW) or/and bortezomib (BTZ) were administered i.v. twice weekly for 2 weeks. Each plot represents the mean+s.d. of tumor volume (n=5). *P<0.05; Steel–Dwass test. (b) Fourteen days after the initial administration, serum M protein concentrations were measured. Each bar represents the mean+s.d. (n=5), *P<0.05; Tukey’s test.
The increased activity of the combination was further confirmed by the change in serum M protein, which was significantly lower in mice treated with KW-2478 and bortezomib in combination than in mice receiving either agent alone as monotherapy (Figure 5b). Importantly, no additional toxicity was observed in the combination group as assessed by treatment-related mortality and body weight change (data not shown).
KW-2478 enhanced anti-tumor activity of bortezomib in orthotopic xenograft model
In order to investigate the anti-tumor effect of KW-2478 and bortezomib in a more clinically relevant model, we used an i.v. inoculated OPM-2/GFP model, which we have described previously.14 Figure 6a shows typical observations in this model. Thirty-three days after OPM-2/GFP cell inoculation, GFP signal was detected and the GFP-fluorescent region was histochemically examined. Inoculated OPM-2/GFP cells developed multi MM lesions in bone marrow, resembling human MM. As shown in Figure 6b, in KW-2478 monotherapy groups at either 100 or 200 mg/kg (groups 2 and 3), no significant decreases of GFP fluorescence intensity were seen. There was a small decrease in GFP-fluorescent lesions in the bortezomib 0.3 mg/kg monotherapy group (group 4), compared with control animals. In the KW-2478 and bortezomib combination groups (Groups 5 and 6), clear decreases in GFP fluorescence intensity were seen compared with the control group, and compared with mice treated with bortezomib or KW-2478 alone. This was also apparent in the lower serum M protein concentrations in mice treated with KW-2478 and bortezomib in combination, compared with those in each monotherapy group, an effect that reached statistical significance with the 200 mg KW-2478 0.3 mg bortezomib group compared with bortezomib alone (Figure 6c). As with NCI-H929 xenograft model, no additive toxicity was observed in the combination group (data not shown).
Anti-tumor activity in OPM-2/GFP orthotopic model. OPM-2/GFP cells (1 × 107 cells/mouse) were inoculated i.v. into severe combined immunodeficient (SCID) mice. (a) Thirty-three days after cell inoculation, GFP fluorescence was measured followed by histochmical examination, (b) Twenty-seven days after cell inoculation, KW-2478 (KW) or/and bortezomib (BTZ) were administered i.v. twice weekly for 2 weeks. Photographs were taken 50 days after cell inoculation from dorsal angle. (c) Forty days after cell inoculation, serum M protein concentration was measured by ELISA. Each column represents the mean+s.d. n=5. *P<0.05; Tukey’s test.
Discussion
Despite recent advances in treatment, MM remains an incurable disease for the majority of patients. To improve the outcome, novel targeted therapies and synergistic combinations with appropriate anti-myeloma agents are urgently needed. In the present study, we showed the beneficial anti-tumor effects of KW-2478 and bortezomib combination in vitro and in vivo using MM cell lines. We could also show increased anti-MM activity with a combination of KW-2478 and bortezomib compared with bortezomib alone in primary MM patient samples.
Previously, it has been reported that the combination of Hsp90 inhibition and proteasome inhibition resulted in enhanced anti-tumor activity.8, 9, 10, 11 The results of our current study are consistent with those previous reports. Although the underlying mechanism of the combinatorial effect is not fully understood, the following mechanism has been proposed. Many cellular proteins are targeted for degradation in the proteasome. When combined with a proteasome inhibitor, Hsp90 inhibition can overload the protein degradation machinery and lead to accumulation of UPR and consequent stress response. As a result, the combined inhibition of both proteasome and Hsp90 induces excessive UPR, including marked induction of Grp78 (BiP) and CHOP and resultant cell death.8 Recently, Roue et al.26 have reported that resistance to bortezomib can be overcome by Hsp90 inhibition with IPI-504, a geldanamycin analog,26 associated with depletion of Grp78. In our present study, however, KW-2478 induced neither accumulation of ubiquitinated proteins nor CHOP (data not shown). Furthermore, KW-2478 had little effect on the level of Grp78 protein. Therefore, the underling mechanism mediating the effects observed in our study may be different from those reported previously. A difference between our study and previous studies are the drug treatment regimen; we adopted 3 h pulse condition while others used continuous treatment. Mechanisms other than Grp78 inhibition or CHOP induction may therefore be important with pulse drug treatments.
Noxa, a BH3-only protein is considered as a key mediator of bortezomib-induced apoptosis. In agreement with previous reports,21, 22, 23 Noxa was induced by exposure to bortezomib in our present study in OPM-2/GFP cells. Noticeably, KW-2478 enhanced bortezomib dependent Noxa induction in a dose-dependent manner. In parallel with the Noxa induction, caspase activation and apoptosis was observed in the MM cells. Therefore, Noxa induction could be one of the mechanisms underlying the combinatorial effects observed. However, in NCI-H929 cells, Noxa induction was not observed, suggesting mechanisms other than Noxa induction also contribute to the activity of the combination. In all the cells tested, OPM-2/GFP, NCI-H929 and hPBMCs, enhanced Hsp70 and/or Hsp70B expression was observed with combination treatments. As Hsp70 family proteins are known to be expressed in response to proteotoxic stress,24, 27 the extensive accumulation of proteotoxic stress is thought to be a basis of the combinatorial effect.
The combination of KW-2478 and bortezomib synergistically enhanced the expression of Hsp70B, a homolog of Hsp70, in not only MM cell line but also hPBMC. Interestingly, such extensive Hsp70B induction was only observed in the combination treatment with both drugs. This result indicated Hsp70B could be a surrogate biomarker to monitor the combinatorial effect. Such a surrogate biomarker would not act as a predictive biomarker of combination activity, but might be useful to monitor the biological activity of the two drugs after treatment. Specifically, if an upregulation of Hsp70B was observed in the PBMCs of a patient after treatment, it would suggest biological activity of the two drugs in combination, before a clinical response was apparent. This may be useful in making decisions about continuing treatment in an individual patient and in selecting the appropriate dosing and administration schedule as this combination is further developed in the clinic.
The combined anti-tumor activity of KW-2478 with bortezomib was evaluated in a NCI-H929 s.c. inoculated model and an OPM-2/GFP orthotopic model, using a twice-weekly administration schedule similar to the clinical administration schedule of bortezomib. In both models, the combination of KW-2478 and bortezomib showed beneficial activities. The enhanced anti-tumor activity observed in the OPM-2/GFP orthotopic model may be particularly informative, as this model is considered to be more relevant to the pathological condition in MM disease. These data indicate that it may be beneficial to add KW-2478 to bortezomib in a clinically practical twice-weekly administration schedule.
The combination of another Hsp90 inhibitor, 17-AAG, and bortezomib has already been investigated in a Phase II study, and has demonstrated significant and durable responses with acceptable toxicity in patients with relapsed and refractory MM.13 As KW-2478 has superior physicochemical and toxicological properties to 17-AAG, the combination of KW-2478 and bortezomib is expected to be more promising. The Phase I/II study of KW-2478 in combination with bortezomib is underway in MM patients.
References
Lonial S, Cavenagh J . Emerging combination treatment strategies containing novel agents in newly diagnosed multiple myeloma. Br J Haematol 2009; 145: 681–708.
Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009; 23: 1152–1157.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Chanan-Khan AA, Borrello I, Lee KP, Reece DE . Development of target-specific treatments in multiple myeloma. Br J Haematol 2010; 151: 3–15.
Rajkumar SV, Richardson PG, Hideshima T, Anderson KC . Proteasome inhibition as a novel therapeutic target in human cancer. J Clinical Oncology 2005; 23: 630–639.
Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res 2007; 67: 1783–1792.
Allegra A, Sant'antonio E, Penna G, Alonci A, D'Angelo A, Russo S et al. Novel therapeutic strategies in multiple myeloma: role of the heat shock protein inhibitors. Eur J Haematol 2011; 86: 93–110.
Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 2007; 110: 2641–2649.
Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood 2006; 107: 1092–1100.
Duus J, Bahar HI, Venkataraman G, Ozpuyan F, Izban KF, Al-Masri H et al. Analysis of expression of heat shock protein-90 (HSP90) and the effects of HSP90 inhibitor (17-AAG) in multiple myeloma. Leuk Lymphoma 2006; 47: 1369–1378.
Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L et al. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci USA. 2006; 103: 17408–17413.
Wright JJ . Combination therapy of bortezomib with novel targeted agents: an emerging treatment strategy. Clin Cancer Res 2010; 16: 4094–4104.
Richardson PG, Badros AZ, Jagannath S, Tarantolo S, Wolf JL, Albitar M et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol 2010; 150: 428–437.
Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y et al. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res 2010; 16: 2792–2802.
Katagiri S, Yonezawa T, Kuyama J, Kanayama Y, Nishida K, Abe T et al. Two distinct human myeloma cell lines originating from one patient with myeloma. Int J Cancer 1985; 36: 241–246.
Gazdar AF, Oie HK, Kirsch IR, Hollis GF . Establishment and characterization of a human plasma cell myeloma culture having a rearranged cellular myc proto-oncogene. Blood 1986; 67: 1542–1549.
Nakashima T, Tanaka R, Yamashita Y, Kanda Y, Hara M . Aranorosin and a novel derivative inhibit the anti-apoptotic functions regulated by Bcl-2. Biochem Biophys Res Commun 2008; 377: 1085–1090.
Chou TC, Talalay P . Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55.
Schmidmaier R, Baumann P, Meinhardt G . Cell-cell contact mediated signalling – no fear of contact. Exp Oncol 2006; 28: 12–15.
Webb JL (ed.) . Effect of more than one inhibitor, antagonism, summation, and synergism. Enzyme Metabolic Inhibitor, Vol 1. Academic Press: New York, 1963, pp 488–512.
Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res 2005; 65: 6282–6293.
Gomez-Bougie P, Wuillème-Toumi S, Ménoret E, Trichet V, Robillard N, Philippe M et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res 2007; 67: 5418–5424.
Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA 2009; 106: 2200–2205.
Noonan EJ, Place RF, Giardina C, Hightower LE . Hsp70B’ regulation and function. Cell stress Chaperones 2007; 12: 393–402.
Hongtao Gu Xiequn, Chen Guangxun, Gao Hongjuan Dong . Caspase-2 functions upstream of mitochondria in endoplasmic reticulum stress-induced apoptosis by bortezomib in human myeloma cells. Mol Cancer Ther 2008; 7: 2298–2307.
Roué G, Pérez-Galán P, Mozos A, López-Guerra M, Xargay-Torrent S, Rosich L et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood 2011; 117: 1270–1279.
Neznanov N, Komarov AP, Neznanova L, Stanhope-Baker P, Gudkov AV . Proteotoxic stress targeted therapy (PSTT): induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget 2011; 2: 209–221.
Acknowledgements
Financial support for this study was provided by Kyowa Hakko Kirin Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ishii, T., Seike, T., Nakashima, T. et al. Anti-tumor activity against multiple myeloma by combination of KW-2478, an Hsp90 inhibitor, with bortezomib. Blood Cancer Journal 2, e68 (2012). https://doi.org/10.1038/bcj.2012.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2012.13
Keywords
This article is cited by
-
In vivo quantitative assessment of therapeutic response to bortezomib therapy in disseminated animal models of multiple myeloma with [18F]FDG and [64Cu]Cu-LLP2A PET
EJNMMI Research (2021)
-
Inhibition of HSP90 overcomes melphalan resistance through downregulation of Src in multiple myeloma cells
Clinical and Experimental Medicine (2020)
-
A lowered 26S proteasome activity correlates with mantle lymphoma cell lines resistance to genotoxic stress
BMC Cancer (2017)
-
A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma
British Journal of Cancer (2017)
-
Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies
British Journal of Cancer (2016)