Abstract
Background:
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is an oncoprotein expressed in several solid cancers. Our purpose was to study its role in serous ovarian cancer patients, and the association to clinicopathological variables and molecular markers.
Methods:
We collected retrospectively 562 consecutive serous ovarian cancer patients treated at the Helsinki University Central Hospital. We stained tumour tissue microarrays for CIP2A by immunohistochemistry and constructed survival curves according to the Kaplan–Meier method. Associations to clinicopathological and molecular markers were assessed by the χ2-test.
Results:
We found strong cytoplasmic CIP2A immunoreactivity in 212 (40.4%) specimens, weak positivity in 222 (42.4%) specimens, and negative in 90 (17.2%). Immunopositive CIP2A expression was associated with high grade (P<0.0001), advanced stage (P=0.0005), and aneuploidy (P=0.001, χ2-test). Cancerous inhibitor of protein phosphatase 2A overexpression was also associated with EGFR protein expression (P=0.006) and EGFR amplification (P=0.043). Strong cytoplasmic CIP2A immunopositivity predicted poor outcome in ovarian cancer patients (P<0.0001, log-rank test).
Conclusion:
Our results show that CIP2A associates with reduced survival and parameters associated with high grade in ovarian cancer patients, and may thus be one of the factors that identify aggressive subtype (type II) of this disease.
Similar content being viewed by others
Main
Ovarian cancer is the sixth most common cancer in women and the second most common gynaecological malignancy in the world. Incidence rates have increased slowly in developed countries, with an incidence rate of 9 per 100 000 (Parkin et al, 2005). Five-year survival is less than 50% for ovarian cancer patients, as most cases are found at an advanced stage (Jemal et al, 2008). The standard treatment is extensive surgery usually followed by chemotherapy.
Ovarian surface epithelium and tubal tissue have been proposed to represent the origins of ovarian cancer, of which the most frequent subtype is serous carcinoma (Dubeau, 2008). Ovarian cancer can be divided into two subgroups: type I tumours that are slowly developing low-grade serous, mucinous, endometriod, and clear-cell carcinomas, whereas type II tumours are rapidly progressing high-grade serous or undifferentiated carcinomas (Levanon et al, 2008). Precursor lesions of the type II ovarian cancers are poorly understood and prognosis is poor. This type of ovarian tumours also more commonly harbour mutations in the p53 gene and show a high proliferation index (Shih and Kurman, 2004; Landen et al, 2008).
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is a human oncoprotein overexpressed in head and neck squamous cell carcinoma and in colon cancer (Junttila and Westermarck, 2007; Junttila et al, 2007; Mumby, 2007). CIP2A interacts with PP2A and prevents PP2A-mediated dephosphorylation of the oncogene c-Myc (Junttila et al, 2007). CIP2A is a marker of reduced overall survival in certain subgroups of gastric cancer (Khanna et al, 2009), and its expression associates with high grade and lymph node metastasis in breast cancer (Come et al, 2009). Its role in ovarian carcinogenesis is, however, unknown. To address the role of CIP2A in ovarian cancer, we investigated the association of CIP2A protein expression to clinicopathological variables and molecular markers in serous ovarian cancer.
Patients and methods
Patients
We collected 562 consecutive patients treated by gynaecological oncologists for serous ovarian carcinoma at the Department of Obstetrics and Gynaecology at the Helsinki University Central Hospital in 1964–2000 (median 1994). The study was approved by the National Supervisory Authority of Welfare and Health. Originally, a gynaecological pathologist examined all specimens, and in addition, another gynaecological pathologist (RB) reviewed them. Staging of the tumours was carried out according to FIGO classification, and grading according to a three-tiered grading system (Silverberg, 2000). Survival data were obtained from patient records and the Population Registry.
Tumour specimens for this study were obtained from primary surgery, and patients received no neoadjuvant treatment. Radical surgery was adopted at the end of 1980s. In 451 of 562 patients (80%), total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed along with surgical removal of tumour masses, together with pelvic and/or para-aortic lymphadenectomy in 175 of these. In all, 54 (10%) patients underwent uni- or bilateral salpingo-oophorectomy, and in 57 (10%) only biopsies were obtained. Before 1990, all patients received chemotherapy according to current praxis. After 1990, all patients except those with stage 1a and b and grade 1 and 2 disease received chemotherapy (Young et al, 1990). Platinum-based chemotherapy served as part of first-line treatment in 404 (72%) cases together with taxanes in 194 (35%).
Response to therapy was evaluated after the initial six cycles of chemotherapy. For those who had no chemotherapy, the evaluation was performed 5–6 months post-surgery. In total, 178 (32%) patients underwent a second-look operation for evaluation of response to treatment (Miller et al, 1981), whereas the rest was based on gynaecological examinations, pelvic ultrasonography, CA-125 measurements, and radiological findings.
Ovarian carcinoma-specific overall survival was calculated from the date of diagnosis to death from ovarian carcinoma. Ovarian carcinoma progression-free survival was calculated for patients who were disease-free after primary treatment (surgery and first-line chemotherapy, if given) from the date of diagnosis to relapse of disease. Median age at diagnosis was 60 years (range 18–92) and median follow-up of patients alive at study end was 8.8 years (range 0.1–41.3). Five-year overall survival rate for the whole cohort was 41.2% (95% confidence interval (CI) 37.1–45.7%).
Preparation of tumour tissue microarrays
Four representative 0.8 mm cores of tumour areas were obtained for each patient using a tissue microarray instrument (Beecher Instruments, Silver Spring, MD, USA), as described (Kononen et al, 1998; Kallioniemi et al, 2001; Torhorst et al, 2001).
Immunohistochemistry
For the detailed immunohistochemistry protocol, see Khanna et al (2009). A rabbit polyclonal CIP2A antibody at a dilution of 1 : 10 000 for 1 h at room temperature served as the primary antibody (Soo Hoo et al, 2002). For validation, we stained a subset (n=95) with an alternative anti-CIP2A antibody (rabbit polyclonal NB100-74663, 1 : 500; Novus Biologicals, Littleton, CO, USA) according to the protocol described. Immunohistochemical analysis for p53 (monoclonal DO-7 antibody, 1 : 100; Dako, Glostrup, Denmark; Lassus et al, 2003), Ki-67 (polyclonal A0047 antibody, 1 : 150; Dako; Lassus et al, 2004), EGFR (mouse monoclonal NCL-EGFR, 1 : 150; Novocastra Laboratories, Newcastle, UK; Lassus et al, 2006), as well as flow cytometry (Jahkola et al, 1998; Lassus et al, 2006) have been described previously.
Scoring of immunoreactivity
Tumour specimens were scored from tissue microarrays independently by CB and AH, blinded to clinical status and outcome data. All specimens were scored and analysed separately for cytoplasmic and nuclear CIP2A immunoreactivity. Cytoplasmic CIP2A immunopositivity was scored 0–3 according to the intensity of cancer cell immunoreactivity, and the highest intensity of the four cores was regarded to represent the final score. Completely negative immunoreactivity was scored as 0 (n=90) and diffuse weak cytoplasmic positivity was 1 (n=222). Moderately positive or focally strongly positive intensity was scored as 2 (n=167) and homogeneously strong intensity was 3 (n=45). Nuclear immunoreactivity was scored as negative (score 0) when <10% of the nuclei stained positive and as positive (score 1) when ⩾10% of the nuclei were positive. Specimens with discordant scores underwent re-evaluation with a multiheaded microscope, and the consensus score served for further analysis. In all, 524 (93%) specimens were scored successfully for CIP2A. In the final analysis, cytoplasmic immunoreactivity was analysed as negative (score 0), weakly positive (score 1), and strongly positive (scores 2 and 3), whereas nuclear immunoreactivity was analysed as negative (score 0) vs positive (score 1).
Cell culture
CaOV3, OVCAR-3 (both from American Type Culture Collection, Manassas, VA, USA), and OV-4 (kindly provided by Dr Timothy J Eberlein, Harvard Medical School, Boston, MA, USA) ovarian adenocarcinoma cell lines were cultured in RPMI-1640 supplemented with 10% foetal calf serum, 2 mM L-glutamine, and antibiotics (Bio Whittaker Europe, Verviers, Belgium), and maintained at 37 °C at 5% CO2 in air.
Protein extraction and immunoblot analysis
Total proteins were extracted in hot Laemmli sample buffer, whereas cytoplasmic and nuclear fractions were prepared with NE-PER nuclear and cytoplasmic extraction kit (Pierce Biotechnology Inc., Rockford, IL, USA). For western blot analysis, 30 μg protein extracts were separated by 12% SDS–PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat milk in TBS–0.1% NP40, and then incubated with the rabbit polyclonal anti-CIP2A (1 : 5000, room temperature, 1 h; Soo Hoo et al, 2002) or goat polyclonal anti-β-actin (1 : 1000, room temperature, 1 h; Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. Subsequently, membranes were incubated with horseradish peroxidase conjugated to goat anti-rabbit (1 : 500; Pierce Biotechnology Inc.) or donkey anti-goat (1 : 2000; Santa Cruz) for 1 h at room temperature. The proteins were visualised with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) or Proteome Grasp ECL Kit (Pierce).
Statistical analysis
The association between CIP2A immunopositivity and clinicopathological variables was assessed by the χ2-test. The correlation between the two different CIP2A antibodies was assessed by the Spearman correlation test. Survival curves were constructed according to the Kaplan–Meier method and were compared with the log-rank test (StatView version StatView for Mac, version 5.0.1; SAS Institute Inc., Cary, NC, USA and SPSS version 17.0 for Mac; SPSS Inc., Chicago, IL, USA). For multivariate survival analysis, the Cox proportional hazard model had the following categorical covariates entered in a backward stepwise manner: FIGO stage (I, II, III, and IV), grade (1, 2, and 3), age at diagnosis (<60 and ⩾60 years), residual tumour size (⩽1 and >1 cm) and cytoplasmic CIP2A expression.
Results
Immunohistochemistry
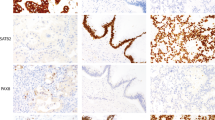
We evaluated CIP2A expression separately for cytoplasmic and nuclear immunoreactivity in 562 serous ovarian cancer specimens, of which 524 (93%) were scored successfully. We found strong cytoplasmic immunopositivity for CIP2A in 212 (40.4%), weak positivity in 222 (42.4%), and negative immunoreactivity in 90 (17.2%) specimens. The cytoplasm of stromal cells remained generally negative. Nuclear CIP2A immunoreactivity was positive in 307 (58.6%) and negative in 217 (41.4%) cases (Figure 1). For validation of the CIP2A antibody, we studied cytoplasmic immunoreactivity with an alternative antibody (NB100-74663; Novus Biologicals), and found a positive correlation between cytoplasmic CIP2A immunopositivity recognised by these two antibodies (rS=0.362, n=95, P<0.0001, Spearman's correlation test).
Association to clinicopathological variables and biomarkers
The associations between clinicopathological variables and CIP2A cytoplasmic and nuclear immunoreactivity are shown in Table 1. Patients with advanced stage (P=0.0005), high grade (P<0.0001), and ascites (P=0.004, χ2-test) presented more frequently with cytoplasmic CIP2A positivity (scores 1–3). CIP2A nuclear positivity was more frequent in young (P=0.015), low-stage patients (P=0.023), in those with low grade (P<0.0001), and in patients free from ascites (P=0.049).
We noted aberrant p53 immunoreactivity (P<0.0001), high proliferation index (Ki-67, P<0.0001), and aneuploidy (P=0.0007) to associate with cytoplasmic CIP2A expression (Table 2). CIP2A associated also with EGFR protein overexpression (P=0.006) and EGFR gene amplification (P=0.043).
Survival analyses
Strong cytoplasmic CIP2A positivity indicated a reduced ovarian cancer-specific 5-year survival of 31.6% (95% CI 24.7–38.4), compared to patients with weak CIP2A positivity with a 5-year survival of 42.4% (95% CI 35.6–49.2), and to those who were negative for cytoplasmic CIP2A 5-year survival of 63.0% (95% CI 52.7–73.3; P=0.0001, log-rank test; Figure 2A). Results were similar for progression-free survival, with 5-year survivals of 39.8% (95% CI 28.8–50.7) for CIP2A strongly positive patients, 52.1% (95% CI 42.9–61.3) for patients with weak CIP2A positivity, and 73.9% (95% CI 62.0–85.8) for CIP2A negative (P=0.0007, log-rank test; Figure 2B). CIP2A nuclear-negative patients had a 5-year ovarian cancer-specific survival of 37.2% (95% CI 30.3–44.2), whereas it was 45.0% (95% CI 39.2–50.8) for those who showed nuclear positivity (P=0.013, log-rank test; Figure 2C).
CIP2A expression and survival in ovarian cancer patients. (A) Overall ovarian cancer-specific survival according to the Kaplan–Meier method (P=0.0001, log-rank test) and (B) progression-free survival (P=0.007, log-rank test) in relation to cytoplasmic CIP2A expression in serous ovarian cancer patients. (C) Overall ovarian cancer-specific survival for nuclear CIP2A expression (P=0.013, log-rank test).
Next, we stratified the survival analysis according to different adjuvant protocols. The 5-year survival for patients who received platinum-based chemotherapy combined with chemotherapeutics other than taxanes (n=194) was 25.2% (95% CI 16.2–34.2) for CIP2A strongly positive patients, 48.3% (95% CI 36.9–59.7) for weakly positive and 64.0% (95% CI 45.2–82.8) for CIP2A negative (P<0.0001, log-rank test; Figure 3A). Among patients who received the currently used platinum-based chemotherapy combined with taxanes (n=188), the 5-year survival for CIP2A strongly positive patients was 43.3% (95% CI 30.6–56.0), 50.8% (95% CI 38.8–62.8) for weakly positive, and 79.0% (95% CI 62.1–95.9) for CIP2A negative (P=0.0241, log-rank test; Figure 3B).
Stratified survival analyses according to adjuvant treatment. (A) Overall ovarian cancer-specific survival for patients who received platinum-based chemotherapy in combination with other chemotherapeutics than taxanes (P<0.0001, log-rank test) and (B) for patients who where treated with the currently used platinum-based chemotherapy combined with taxanes (P=0.0241, log-rank test).
Multivariate survival analysis
We performed multivariate survival analysis for, in this material, previously independent prognostic factors (age, grade, stage, residual tumour size, and aberrant p53 immunoreactivity) (Lassus et al, 2003; Erkinheimo et al, 2004). When we included cytoplasmic CIP2A expression into Cox multivariate analysis, the hazard ratio, with CIP2A-negative patients as reference, was 1.31 (95% CI 0.89–1.95) for CIP2A weakly positive and 1.20 (95% CI 0.80–1.79) for strongly positive (P=0.358).
Cytoplasmic and nuclear CIP2A expression
The cellular sublocalisation of CIP2A protein was studied in the ovarian adenocarcinoma cell lines CaOV3, OVCAR-3, and OV-4. We noted that CIP2A protein is highly expressed in both the cytoplasmic and nuclear compartments (Figure 4).
CIP2A protein expression in ovarian cancer cells. In western blot analysis, CIP2A protein is expressed both in cytoplasmic and nuclear protein fractions in the CaOV3, OVCAR-3, and OV-4 ovarian adenocarcinoma cells. Lamin was used as nuclear-positive control and β-tubulin as cytoplasmic-positive control.
Discussion
In this study, we found that strong cytoplasmic expression of CIP2A in ovarian cancer patients is a marker of reduced overall and progression-free survival. This is in line with our previous results in gastric cancer, where we showed that CIP2A expression associates with reduced survival in the subgroups of small tumours and p53-immunopositive tumours (Khanna et al, 2009), and in tongue cancer, where we demonstrated that CIP2A serves as an independent marker of reduced survival (Böckelman et al, 2011). Dong et al (2010) have similarly demonstrated that CIP2A expression associates with reduced survival non-small-cell lung cancer, which was not, however, the case in another study focused on breast cancer (Come et al, 2009). In multivariate survival analysis in our current serous ovarian cancer material, CIP2A expression did not demonstrate independent prognostic value. All previous studies concerning CIP2A expression in tumours have focused on cytoplasmic expression. We scored cytoplasmic and nuclear expression separately and found that for nuclear CIP2A expression, the results with regard to survival were opposite compared with cytoplasmic expression, as negative nuclear expression of CIP2A indicated poor outcome. When CIP2A was first recognised as p90, Soo Hoo et al (2002) demonstrated its localisation to the perinuclear regions of the cytosol. Junttila et al (2007) noted its overexpression with predominant cytoplasmic localisation and only weak nuclear expression in head and neck squamous cell carcinoma and colon cancer. Recent studies have only addressed the cytoplasmic role of CIP2A, and the biological function of nuclear CIP2A is largely unknown. This raises an important issue, which calls for studies about the functional significance of nuclear CIP2A. In ovarian cancer cell lines, CIP2A protein was expressed to a high extent in both cytoplasmic and nuclear protein fractions. Our findings suggest that nuclear CIP2A protein may have a, to date not yet determined, functional role in ovarian carcinogenesis.
Previous studies have suggested distinct molecular pathogenesis and clinical manifestation for different histological types (Kobel et al, 2008), and hence, we decided to limit our study to serous histological type. Our clinical material is relatively large with a long follow-up time, unfortunately reflected by our patients being treated with heterogeneous treatment modalities. A significant proportion of the patients (n=188), however, received the currently used platinum-based therapy in combination with taxanes. We found that also among these patients, CIP2A was a marker of poor outcome, demonstrating that the prognostic role of CIP2A is maintained also in the patient subgroup receiving current adjuvant treatments.
Ovarian cancer has been proposed to evolve through two distinct molecular pathways: type I low-grade pathway tumours have a 5-year survival of 55% and have frequently activating mutations of BRAF or KRAS, whereas type II high-grade pathway tumours with a 5-year survival of only 30% are characterised by inactivating mutations of p53. According to this hypothesis, hallmarks of the type II pathway are high grade, high proliferation index, and p53 mutations (Shih and Kurman, 2005; Singer et al, 2005). In this patient material, we have previously shown that aberrant p53 expression is an independent predictor of poor survival and that it is associated with clinicopathological indicators of aggressive tumour behaviour (Lassus et al, 2003). Interestingly, the 5-year survival for patients with cytoplasmic CIP2A overexpressed (32%) was similar to the 5-year survival of the type II ovarian tumours (30%). We found here that cytoplasmic CIP2A positivity was associated with aggressive disease characteristics, namely high grade, advanced stage, high proliferation index, aneuploidy, and aberrant p53 immunoreactivity. Similarly, CIP2A expression was associated with high proliferation index and aneuploidy in our study on gastric cancer (Khanna et al, 2009), and in breast cancer, it was reported to associate with proliferation index, p53 mutation, and high tumour grade (Come et al, 2009). Taken together, these results propose that cytoplasmic CIP2A expression is a marker of a rapidly growing and aggressive disease.
Zhao et al (2010) investigated the role of cagA-positive Helicobacter pylori on CIP2A expression in gastric cancer, and found that the CagA-induced upregulation of CIP2A is mediated through the MEK/ERK pathway. Khanna et al (2011) continued this hypothesis by showing that the MEK1/2 and EGFR inhibitors inhibit CIP2A expression, whereas activation of MEK1/2–ERK signalling pathway stimulates CIP2A expression. They established the ETS1 transcription factor as the mediator of the EGFR–MEK1/2–ERK-induced positive regulation of CIP2A. Our association of CIP2A expression with EGFR protein expression and EGFR gene amplification could provide one putative mechanism for the regulation of CIP2A in human ovarian cancer.
In conclusion, our results demonstrate that overexpression of cytoplasmic CIP2A in serous ovarian cancer serves as an indicator of poor overall and progression-free survival. Associating with markers of aggressive disease, it may play a role in the type II serous ovarian cancer pathway.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Böckelman C, Hagström J, Mäkinen LK, Keski-Säntti H, Häyry V, Lundin J, Atula T, Ristimäki A, Haglund C (2011) High CIP2A immunoreactivity is an independent prognostic indicator in early-stage tongue cancer. Br J Cancer 104: 1890–1095
Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, Kallioniemi O, Thezenas S, Westermarck J (2009) CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res 15: 5092–5100
Dong QZ, Wang Y, Dong XJ, Li ZX, Tang ZP, Cui QZ, Wang EH (2010) CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann Surg Oncol 18: 857–865
Dubeau L (2008) The cell of origin of ovarian epithelial tumours. Lancet Oncol 9: 1191–1197
Erkinheimo TL, Lassus H, Finne P, van Rees BP, Leminen A, Ylikorkala O, Haglund C, Bützow R, Ristimäki A (2004) Elevated cyclooxygenase-2 expression is associated with altered expression of p53 and SMAD4, amplification of HER-2/neu, and poor outcome in serous ovarian carcinoma. Clin Cancer Res 10: 538–545
Jahkola T, Toivonen T, Virtanen I, von Smitten K, Nordling S, von Boguslawski K, Haglund C, Nevanlinna H, Blomqvist C (1998) Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer 78: 1507–1513
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58: 71–96
Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Bottzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, Lu SL, Lin S, Chan EK, Wang XJ, Grenman R, Kast J, Kallunki T, Sears R, Kahari VM, Westermarck J (2007) CIP2A inhibits PP2A in human malignancies. Cell 130: 51–62
Junttila MR, Westermarck J (2007) Mechanisms of MYC stabilization in human malignancies. Cell Cycle 7: 592–596
Kallioniemi OP, Wagner U, Kononen J, Sauter G (2001) Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet 10: 657–662
Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C, Westermarck J, Ristimäki A (2009) MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst 101: 793–805
Khanna A, Okkeri J, Bilgen T, Tiirikka T, Vihinen M, Visakorpi T, Westermarck J (2011) ETS1 mediates MEK1/2-dependent overexpression of cancerous inhibitor of protein phosphatase 2A (CIP2A) in human cancer cells. PLoS One 6: e17979
Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, Santos J, Swenerton K, Gilks CB, Huntsman D (2008) Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5: e232
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4: 844–847
Landen CN, Birrer MJ, Sood AK (2008) Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol 26: 995–1005
Lassus H, Leminen A, Lundin J, Lehtovirta P, Bützow R (2003) Distinct subtypes of serous ovarian carcinoma identified by p53 determination. Gynecol Oncol 91: 504–512
Lassus H, Leminen A, Väyrynen A, Cheng G, Gustafsson JA, Isola J, Bützow R (2004) ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol Oncol 92: 31–39
Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN, Bützow R (2006) Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med 84: 671–681
Levanon K, Crum C, Drapkin R (2008) New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 26: 5284–5293
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 207–214
Mumby M (2007) PP2A: unveiling a reluctant tumor suppressor. Cell 130: 21–24
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Shih I, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164: 1511–1518
Shih I, Kurman RJ (2005) Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res 11: 7273–7279
Silverberg SG (2000) Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol 19: 7–15
Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih I (2005) Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol 29: 218–224
Soo Hoo L, Zhang JY, Chan EK (2002) Cloning and characterization of a novel 90kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene 21: 5006–5015
Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, Mross F, Dieterich H, Moch H, Mihatsch M, Kallioniemi OP, Sauter G (2001) Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 159: 2249–2256
Young RC, Walton LA, Ellenberg SS, Homesley HD, Wilbanks GD, Decker DG, Miller A, Park R, Major F (1990) Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med 322: 1021–1027
Zhao D, Liu Z, Ding J, Li W, Sun Y, Yu H, Zhou Y, Zeng J, Chen C, Jia J (2010) Helicobacter pylori CagA upregulation of CIP2A is dependent on the Src and MEK/ERK pathways. J Med Microbiol 59: 259–265
Acknowledgements
CIP2A antibody was a kind gift from Dr Edward K Chan, University of Florida (Orlando, FL, USA). We thank Anne Aarnio, Tuire Koski, and Päivi Peltokangas for their excellent technical assistance. This work was financially supported by the Academy of Finland (Project No. 114899 to AR; Project No. 1121413 to JW), Emil Aaltonen Foundation (JW), Finska Läkaresällskapet (CH), Finnish Cancer Society (AR, JW), Foundation for the Finnish Cancer Institute (JW), Helsinki University Central Hospital Research Funds (AR), Kurt och Doris Palander Foundation (CB), Medicinska understödsföreningen Liv och Hälsa (CH), Sigrid Jusélius Foundation (CH, JW and AR), Waldemar von Frenckell Foundation (CB), and Weikko Wilhelm Peltonen Foundation (CB).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Böckelman, C., Lassus, H., Hemmes, A. et al. Prognostic role of CIP2A expression in serous ovarian cancer. Br J Cancer 105, 989–995 (2011). https://doi.org/10.1038/bjc.2011.346
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.346
Keywords
This article is cited by
-
Genomic regulation of transcription and RNA processing by the multitasking Integrator complex
Nature Reviews Molecular Cell Biology (2023)
-
Prodigiosin inhibits the proliferation of glioblastoma by regulating the KIAA1524/PP2A signaling pathway
Scientific Reports (2022)
-
CIP2A regulates MYC translation (via its 5′UTR) in colorectal cancer
International Journal of Colorectal Disease (2021)
-
CIP2A mediates fibronectin-induced bladder cancer cell proliferation by stabilizing β-catenin
Journal of Experimental & Clinical Cancer Research (2017)
-
Overexpression of CIP2A is associated with poor prognosis in multiple myeloma
Signal Transduction and Targeted Therapy (2017)