Abstract
Background:
This retrospective register study assessed overall survival (OS) and influential factors on OS in Swedish renal cell carcinoma (RCC) patients.
Methods:
Using three merged national health registers, Cox proportional-hazards analysis was conducted and, in three models, it was used to assess the impact of cytokine (interferon-α and tyrosine kinase inhibitor (TKI; sunitinib or sorafenib) treatment on OS in metastatic (m)RCC.
Results:
From 2000 to 2008, 8009 patients were diagnosed with RCC and 2753 with mRCC (2002–2008). Median OS in RCC patients diagnosed from 2006 to 2008 compared with 2000–2005 was not reached vs 47.9 months (P<0.001), and in mRCC patients diagnosed from 2006 to 2008 compared with 2002–2005, was 12.4 vs 9.6 months, respectively (P=0.004). Factors associated with significantly improved OS in RCC were female gender, lower age, and previous nephrectomy, and, in mRCC female gender, previous nephrectomy, and any TKI prescription (Model 1: median-adjusted OS, 19.4 months (TKI patients) vs 9.7 months (non-TKI patients); hazard ratio, 0.621; P<0.001).
Conclusion:
OS was improved in Swedish patients diagnosed with RCC and mRCC in the period 2006–2008 compared with 2000–2005 (RCC) and 2002–2005 (mRCC). Although multifactorial in origin, results suggest that increased nephrectomy rates and the use of TKIs contributed to the improvement seen in mRCC patients.
Similar content being viewed by others
Main
Introduction of modern targeted therapies has greatly improved the prognosis of patients with metastatic renal cell carcinoma (mRCC). The multitargeted tyrosine kinase inhibitors (TKIs) sunitinib and sorafenib were the first new therapies approved for advanced RCC and have been available in the European Union since 2006. In a clinical trial of treatment-naive mRCC patients, sunitinib significantly improved progression-free survival (PFS) vs interferon-α (IFN-α; median PFS, 11 vs 5 months, P<0.001; Motzer et al, 2007) and was associated with an improved median overall survival (OS) of 26.4 and 21.9 months (P=0.051), respectively (Motzer et al, 2009). In another trial, sorafenib significantly improved PFS vs placebo in patients after failure of one systemic therapy (cytokine in >80% of cases); median PFS was 5.5 vs 2.8 months, respectively (P<0.01; Escudier et al, 2007). OS was similar in the sorafenib and placebo groups (17.8 vs 15.2 months, respectively; P=0.146), although the results were confounded by extensive cross-over (Escudier et al, 2009a). Despite impressive clinical trial results, there is a paucity of survival data from population-based studies reflecting these advances in clinical practice.
Sweden has a long heritage in maintaining public health registries and is therefore uniquely placed as a source of comprehensive and high-quality epidemiological data. The Swedish Cancer Register, for example, has existed since 1958 and holds information on almost all patients with a cancer diagnosis (Barlow et al, 2009).
The RENal COMParison (RENCOMP) study was a retrospective, non-interventional study using data from the Swedish Cancer Register and two other national registries. The objectives were to assess temporal and regional trends in treatment patterns and survival of Swedish patients with RCC and to describe the impact of resource use.
The objectives of the RENCOMP analyses reported here were to assess changes in OS in Swedish patients with RCC and mRCC by comparing these nationwide patient cohorts diagnosed before and after the introduction of targeted therapies. We also investigated factors influencing OS in both populations and assessed the impact of first-line treatment (sunitinib, sorafenib, or IFN) and treatment sequences in mRCC.
Materials and methods
Study design and patients
This retrospective study used data from Swedish patients compiled in three registries by the National Board of Health and Welfare, Stockholm, Sweden (Table 1). Patients aged ⩾16 years with a diagnosis of RCC (malignant neoplasm of the kidney, except renal pelvis) between 1958 and 2008 were identified by review of the Swedish Cancer Register for the International Classification of Diseases and Related Health Problems (ICD)-7 diagnosis codes I800 or I809 (excluding I801 (cancer of the renal pelvis)); these codes are equivalent to ICD-10 codes C64.0 and C64.9. The RCC study population, including mRCC patients, was limited to patients diagnosed from 2000 to 2008 to reflect modern RCC treatment. The mRCC population comprised patients diagnosed with metastatic disease (see below) from 2002 to 2008, including a number of patients with an RCC diagnosis before the year 2000. Cohorts for comparison purposes were defined as patients (with mRCC) diagnosed from 2000 (2002) to 2005 vs 2006–2008. For these patients, data from the Swedish Cancer Register were merged with data from the National Patient Register (December 2009) and Swedish Prescribed Drug Register (July 2005–October 2010) using personal identification numbers. Dates of death were updated as of October 2010. All data were made anonymous before use in the analysis.
As the Cancer Register did not include full information on the date for mRCC diagnosis, the mRCC population was defined based on a derived date for diagnosis of metastatic disease. The date was derived as when one of three events (whichever occurred first) was recorded in the registry database. These events comprised (1) diagnosis of primary metastasis (M1 disease); (2) diagnosis of secondary (malignant) tumour; and (3) the first visit to an oncology clinic. These criteria could be properly applied from 2002 onwards, which explains why the RCC and mRCC cohorts differed in terms of years included.
TKI medication was defined as any medication dispensed after the date of diagnosis of mRCC with an Anatomical Therapeutic Classification (ATC) code L01XE04 (sunitinib) or L01XE05 (sorafenib); cytokine medication was defined as any medication dispensed after the date of mRCC diagnosis with an ATC code L03AB (includes different types of alpha-IFNs approved for RCC, and well as IFN-β and IFN-γ) or L03AC (includes IL-2 approved for RCC, as well as IL-11). Importantly, there is a large fraction of non-prescription TKI use in Sweden (on average, >40% of prescriptions made according to sales figures, with regional differences, and slowly increasing over time), in which TKIs are dispensed at clinics and not recorded in the Prescribed Drug Register.
Statistical methods
All data were presented using descriptive statistics, that is, frequency and relative frequency for categorical variables, and mean and s.d. and/or median and minimum and maximum for continuous variables. Median OS (reported in months, in which 1 month=30 days) was estimated by Kaplan–Meier method and compared between patients with RCC and, separately, between patients with mRCC, by year and period of diagnosis using the log-rank test for univariate analysis. Multivariate regression analysis was performed using the Cox proportional-hazards model. The regression model for OS in the overall RCC population, as well as models for OS in those with mRCC, included the following covariates: period of diagnosis, age, gender, institution size, nephrectomy status, and geographic region.
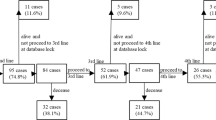
Three different Cox proportional-hazards models were used for analysis of OS in the cohort of patients with mRCC. mRCC Model 1 assessed the effect (hazard ratio (HR)) of at least one TKI prescription (compared with no TKI prescription) on OS; Model 2 examined the impact of first-line treatments (sunitinib, sorafenib, or IFN) as separate categories in the analysis of OS, which could include a subsequent treatment (Figure 1); and Model 3 investigated the effect of various treatment sequences on OS in mRCC patients (Figure 1). The reference category, that is, comparator, for the HR estimation of first-line treatment and treatment sequences in Models 2 and 3 included patients with no RCC drug treatment or other treatments (non-cytokine/non-TKI). From the results of the multivariate regression analyses, median OS was estimated to describe the differences of interest in Models 1–3.
All tests were two-sided and P<0.05 was regarded as a statistically significant result. PASW Statistics v18 (IBM SPSS Statistics; IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
Patients
Between 2000 and 2008, 8009 patients were diagnosed with RCC, comprising the RCC study population, of whom 533 (7%) were diagnosed at autopsy. Between 2002 and 2008, 2753 patients, representing 44% of patients diagnosed with RCC during the same period, met the necessary criteria for a derived diagnosis of mRCC and comprised the mRCC study population. These included patients who presented with metastatic disease at initial diagnosis (n=872; 31% of patients with a derived mRCC diagnosis); patients diagnosed with metastatic disease by means of a secondary malignant tumour, subsequent to diagnosis of localised RCC (n=1311; 48%); and patients diagnosed based on their first visit to an oncologist (n=570; 21%).
Mean (s.d.) age at diagnosis of RCC and mRCC was 70 (12) and 69 (11) years, respectively (with 16% of mRCC patients aged ⩾80 years). In all, 60% and 61% were male, respectively, and 39% of both RCC and mRCC patients were diagnosed in university clinics. Seventy-five percent of RCC patients who did not develop metastases during the observation period (80% in the period 2006–2008) and 60% of patients with M1 disease (by 2008) had a record of nephrectomy.
Patient characteristics for the RCC and mRCC study populations and the cohorts for period of diagnosis are listed in Table 2.
Drug treatment patterns in mRCC
Between July 2005 (the earliest year that prescription data were available) and October 2010, among patients diagnosed with mRCC from 2002 to 2008 (n=2753), 759 (28%) were dispensed a prescription for this condition, and 417 patients (15%) were dispensed at least one TKI either as first- or second-line treatment after another TKI or IFN. Of those patients diagnosed with mRCC from 2006 to 2008 (n=1217), 513 patients (42%) were dispensed a prescription and 360 (30%) were dispensed a TKI. The number of patients treated in first- or second-line with sunitinib or sorafenib, as well as IFN, is shown in Figure 1. Of the total 2002–2008 mRCC population, based on prescription, 8% of patients with mRCC received sunitinib as first-line treatment, 10% received first-line IFN, and 4%, first-line sorafenib; however, most patients (78%) received other or no treatment. These seemingly small shares depend on the availability of prescription data (July 2005–October 2010), introduction of TKIs in late 2006, and non-prescription TKI use as described above. Thirty-five percent of patients treated in first line received a second-line treatment, and only 28% received another TKI after failing one previous TKI. Other treatments included tamoxifen, oral chemotherapeutic drugs, and other hormonal therapy.
Overall survival
RCC population
Between 2000 and 2008, 3- and 5-year OS rates for the total RCC population were 58% and 49%, respectively. Among all RCC patients diagnosed during this period (N=8009), 4181 (52%) died on or before October 2010, and median (unadjusted) OS, estimated by the Kaplan–Meier method, was 1816 days (i.e., 60.5 months (95% CI: 56.1–64.9)).
There was a statistically significant incremental improvement in median OS over time, as estimated by Kaplan–Meier method, in RCC patients diagnosed between 2000 and 2008 (Figure 2A), and in RCC patients diagnosed in the period 2006–2008 compared with 2000–2005, in which median OS was not reached vs 47.9 months (95% CI: 43.2–52.7), respectively (P<0.001; Figure 2B).
When controlling for confounding factors in the multivariate analysis, RCC patients diagnosed from 2006 to 2008 showed 28.6% reduction in the risk of death compared with those diagnosed from 2000 to 2005 (HR, 0.714, 95% CI: 0.664–0.767; P<0.001; Table 3), as reflected in median OS (not reached vs 46.7 months, respectively). Other factors associated with a significantly reduced risk of death in the total RCC population included female gender, lower age, previous nephrectomy, and treatment in the Stockholm, southern, and eastern regions, as compared with the western region of Sweden (Table 3).
mRCC population
For patients diagnosed with mRCC between 2002 and 2008, 3- and 5-year survival rates for the total population were 21% and 13%, respectively, and the median (unadjusted) OS, estimated by the Kaplan–Meier method, was 10.9 months (95% CI: 10.0–11.8). There was a statistically significant incremental improvement in median OS over time, as estimated by Kaplan–Meir method, in mRCC patients diagnosed between 2002 and 2008 (Figure 3A). For mRCC patients diagnosed in the periods 2006–2008 and 2002–2005, median OS was 12.4 months (95% CI: 11.0–13.8) vs 9.6 months (95% CI: 8.5–10.7), respectively (P=0.004; Figure 3B).
When controlling for confounding factors in the multivariate analysis, the risk of death was not statistically significantly different between mRCC patients diagnosed from 2006 to 2008 compared with those diagnosed from 2002 to 2005 (HR, 1.029, 95% CI: 0.941–1.125; P=0.536; Table 4; mRCC Model 1), as reflected in the median-adjusted OS (10.6 vs 11.1 months, respectively). This finding was mainly explained by nephrectomy rates and use of TKIs (Supplementary Table S1). Accordingly, prescription of any TKI was associated with a significantly reduced risk of death (HR, 0.621, 95% CI: 0.547–0.705; P<0.001; Table 4) for which the median-adjusted OS was 19.4 months for TKI patients vs 9.7 months for non-TKI patients (Figure 4).
According to mRCC Model 2, which evaluated the first-line treatment with sorafenib, IFN, and sunitinib, irrespective of subsequent treatment, all first-line treatment options were associated with a statistically significantly reduced risk of death (HR, 0.682–0.596; P⩽0.001; Table 4) corresponding to a median-adjusted OS of 16.1 months with sorafenib to 19.6 months with sunitinib vs 9.2 months with other/no treatment (Figure 5).
mRCC Model 3, which investigated the effect of various treatment sequences on OS, confirmed a similar reduction in risk of death for patients receiving these sequences as compared with patients who received other or no treatment (Table 4). However, for patients who received one treatment solely, only sunitinib and IFN were associated with a statistically significantly reduced risk of death as compared with other or no treatment (Table 4).
Other factors associated with significantly reduced risk of death, and common to all mRCC models, included female gender, previous nephrectomy, and treatment in the Stockholm region, as compared with the western region of Sweden (Table 4).
Discussion
By analysing three different merged national registries in Sweden, we had a unique opportunity to evaluate survival in patients diagnosed with RCC and mRCC before and after the introduction of targeted therapies in 2006. Specifically, we were able to demonstrate the improvements in survival that occurred in Swedish patients diagnosed with mRCC after the introduction of TKIs. The two RCC cohorts used for comparison had similar characteristics at diagnosis (Table 2), with only the following trends noted: an increase in the rate of nephrectomy, a decline in the mean age at diagnosis, and a slight stage migration, mainly from stage T2 to T1. Although these trends indicate a more active approach to the disease, these small differences alone cannot explain the differences in the survival between the two cohorts. We also showed that TKI treatment together with an increased nephrectomy rate were two important factors behind the variation in observed OS in the mRCC cohorts. This was demonstrated by an almost 10-month survival benefit among TKI-treated patients compared with patients who received non-TKI or no therapy.
The influence of other factors associated with OS was also analysed. For example, female gender was associated with lower risk of death (longer OS), an effect previously documented that may be related to less advanced disease at presentation in female patients (Aron et al, 2008; Woldrich et al, 2008). As expected, previous nephrectomy, the standard of care for patients with localised disease, was also associated with lower risk of death (longer survival). The impact of cytoreductive nephrectomy is well established in patients with RCC treated with IFN (Flanigan et al, 2004) and also retrospectively demonstrated with targeted therapy (Choueiri et al, 2011), although it tends to be used only in patients with better prognosis. In our study, the impact of nephrectomy, in terms of HR, was more marked in the overall RCC population than in mRCC patients, perhaps reflecting the potentially curative nature of surgery in early stage RCC.
Median unadjusted OS of the RCC population in our study, as estimated by Kaplan–Meier method, was 60.5 months, with a 5-year survival rate of 49%, both of which are less than the figures reported by the US SEER database (median OS 110 and 130 months for males and females, respectively, with 5-year survival rates of 65% and 69%, respectively; Aron et al, 2008). These differences may be explained by the younger age at diagnosis in the SEER population compared with the age at diagnosis of patients in our study (mean, 62 vs 70 years, respectively) and a slightly smaller proportion of patients presenting with M1 disease at diagnosis (9.9% vs 27%, respectively). More recent SEER data for the early targeted therapy era (2004–2007) showed a crude 3-year survival rate of 75% compared with 68% for the cytokine era (1998–2003), again with younger patients and fewer presenting with distant metastasis, which may account for the observed differences (Shek et al, 2012); in our study, 3-year survival was estimated at 58%. However, it should be noted that patients diagnosed at autopsy were included in our analysis (7%), shifting the survival curve downward.
Median unadjusted OS of the total mRCC population in our study was 10.9 months, as estimated by Kaplan–Meier method, 9.6 months in the 2002–2005 cohort and 12.4 months in the 2006–2008 cohort. These figures might seem low in comparison to published series on treated patients with cytokines and TKIs (Coppin et al, 2005; Gore et al, 2009; Stadler et al, 2010) but represent a population-based analysis including a large number of non-treated and poorly performing patients. It is also likely that the derived date of mRCC diagnosis in patients was set later than the actual date, as it was based on an estimate (algorithm) rather than an observed date. This suggests caution when interpreting and indirectly comparing median survival estimates across studies, while HRs likely provide a more accurate assessment of treatment benefit.
Nearly all of the three treatments analysed, alone or used in sequences, were associated with improved OS compared with other or no treatment. The only exception was sorafenib used as first-line treatment followed by best supportive care, although patient numbers were low. Whether this implies inferior efficacy with sorafenib as first-line treatment compared with other TKIs, as previously shown in a randomised phase II trial (Escudier et al, 2009b), cannot be concluded with this study; however, the data suggest that sorafenib efficacy, in terms of OS, improves when used in sequence. By contrast, our findings related to sunitinib treatment appear to justify its recommendation as first-line treatment for mRCC in both European and US guidelines (Ljungberg et al, 2010; Motzer et al, 2011). mRCC Model 3 seemed to suggest an equal improvement in OS regardless of sequence, which is consistent with a recent Czech register study and German phase II study (Herrmann et al, 2011; Buchler et al, 2012). However, an Italian retrospective study suggested a benefit for the sorafenib–sunitinib sequence compared with sunitinib–sorafenib (Porta et al, 2011). A definitive answer regarding the most clinically beneficial sequence most likely requires a phase III trial, specifically designed to address this question; such a trial is currently ongoing in Germany, with results due in 2013 (NCT00732914). Regardless, consistent with findings by Heng et al (2010), a low fraction of patients in this study (28%) received second-line TKI treatment after failure of another TKI. Even if these data represent an early treatment era, this rate is substantially lower than might be expected by clinicians and clearly emphasises the importance of carefully selecting first-line treatment.
Regional differences were present in the analysis, consistent with findings in other therapeutic areas in Sweden (Swedish National Board of Health and Welfare, 2011). However, it is possible that other factors than those corrected for in the analysis could explain these regional differences.
There were several limitations to our study. One was that the multivariate analyses did not include important RCC prognostic factors, such as performance status, risk group stratification based on published Memorial Sloan–Kettering Cancer Center data (Motzer et al, 2002), or histology, as these data are not recorded in the registers. However, the lack of these data was not expected to affect the OS estimates in the Kaplan–Meier analysis as there is no reason to expect that the risk profile distribution changed over time.
Most importantly, however, this was a retrospective study, indicating a substantial risk of bias. Although selection bias is certainly a problem in the nephrectomy decision in mRCC, a clear selection strategy had not been established for TKI treatment in the early years captured in this study, as physicians were learning the effects of these drugs. There is a reasonable possibility of some selection bias with regard to performance status that could overestimate the effects of TKI treatment. On the other hand, information bias resulting from the fact that the study population included a large fraction of patients with oral drugs dispensed at clinics or patients on intravenous treatment, such as temsirolimus or bevacizumab (also dispensed at clinics), as well as patients treated with IFN before 2005, introduced misclassification of patients as having received other or no treatment. Although these omissions did not affect OS estimates in the Kaplan–Meier analyses (including the temporal comparisons), sensitivity analysis showed that the omissions affected the multivariate analysis by underestimating the importance of TKI use and blurring the impact of IFN; specifically, selection bias with regard to performance status and combinations with bevacizumab in the later period may have overestimated treatment effects, especially when used in sequence, while IFN prescribed before 2005 is categorised as other/no treatment and likely underestimates the effects of treatment. This implies that some graphs, such as Figures 4 and 5, should be interpreted with caution; however, biases to some extent antagonise each other, making a true difference in terms of OS likely. Finally, the algorithm used to determine which patients had metastatic disease and the date of mRCC diagnosis has its flaws and we cannot exclude the possibility that (1) non-metastatic patients were included in the analysis and/or (2) some metastatic patients were excluded, despite a thorough optimisation process. Initially, a fourth criterion was used to define the derived date of mRCC diagnosis, namely if, secondary to an RCC diagnosis, a patient visited a urology department ⩾4 times within a 12-month period. (In Sweden, it is not uncommon for patients living far from oncology clinics to be cared for in a surgical clinic.) Use of this criterion captured patients with a markedly better survival in later years and only a minority of confirmed mRCC patients. Sensitivity Kaplan–Meier and multivariate analyses (Supplementary Figure S1 and Supplementary Table S1), which incorporated this fourth criterion, clearly represented an overestimation of the treatment effects due to a high number of non-metastatic patients. This criterion was therefore omitted from the primary analysis. Furthermore, because the M1 criterion was not available in the Cancer Register from 2000 to 2001 (no registration) and no outpatient visit registration was made during 2000, it was decided to refrain from using patients from these years, as they did not appear to truly reflect a similar composition of mRCC patients, as compared with the other years. This likely means that the results reported here are conservative but consistent and regarded as the best achievable. The true crude OS estimates most probably lie somewhere in between the two models (Figure 3 and Supplementary Figure S1).
In conclusion, the results from our retrospective study are encouraging, consistent with findings from previously reported real-life register studies (Heng et al, 2009; Shek et al, 2012), and would appear to justify the optimism stemming from use of targeted agents. In addition, this provides verification of treatment benefit in a general population beyond clinical trials – evidence that is sparse in the mRCC literature. Specifically, we showed that the risk of death was lowered, that is, OS was improved, in Swedish patients diagnosed with RCC and mRCC, between 2006 and 2008 compared with before 2006, findings that are consistent with recent developments in diagnostic and therapeutic approaches for RCC. Although the observed increase in OS is multifactorial in origin, including improved palliative care, a contribution of targeted therapies is highly probable, as evidenced by the results in which prescription of any TKI was associated with improved OS in mRCC patients. However, the data represent the early years of TKI treatment in Sweden, primarily with sunitinib and sorafenib. More widespread use of targeted agents, the existence of additional treatments not available in this retrospective study (e.g., everolimus and pazopanib), as well as increased clinician experience, may result in further improved patient outcomes. Although the availability of new mRCC agents has not, as far as we know, provided a cure for patients, it has offered the potential to qualitatively extend life two to three times longer than was possible with cytokine-based regimens (Coppin et al, 2005). Furthermore, the added value of these agents’ ability to shrink growing tumours and relieve tumour-related symptoms should not be underestimated from a quality-of-life perspective. Finally, our study highlights the value of health registers, as well as the limitations of real-life data analysis, underscoring the importance of prospective data collection.
References
Aron M, Nguyen MM, Stein RJ, Gill IS (2008) Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol 54: 133–140
Barlow L, Westergren K, Holmberg L, Talbäck M (2009) The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 48: 27–33
Buchler T, Klapka R, Melichar B, Brabec P, Dusek L, Vyzula R, Abrahamova J (2012) Sunitinib followed by sorafenib or vice versa for metastatic renal cell carcinoma--data from the Czech registry. Ann Oncol 23: 395–401
Swedish National Board of Health and Welfare, 2009 Cancer Incidence in Sweden 2009. Available at http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18204/2010–12–17.pdf accessed 31 August 2011
Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, McDermott DF, Rini BI, Heng DY (2011) The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 185: 60–66
Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T (2005) Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev 1: CD001425
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM TARGET Study Group (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM (2009a) Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27: 3312–3318
Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM (2009b) Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 1280–1289
Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED (2004) Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 171: 1071–1076
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano D, Vrdoljak E, Schöffski P, Mainwaring P, Nieto A, Yuan J, Bukowski R (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10: 757–763
Heng DY, Chi KN, Murray N, Jin T, Garcia JA, Bukowski RM, Rini BI, Kollmannsberger C (2009) A population-based study evaluating the impact of sunitinib on overall survival in the treatment of patients with metastatic renal cell cancer. Cancer 115: 776–783
Heng DY, Xie W, Bjarnason GA, Vaishampayan UN, Donskov F, Wood L, Knox JJ, Tan M, Kollmannsberger CK, Rini BI, Choueiri TK (2010) A unified prognostic model for first- and second-line targeted therapy in metastatic renal cell carcinoma (mRCC): results from a large international study. J Clin Oncol 28: 15s suppl; abstr 4523
Herrmann E, Marschner N, Grimm MO, Ohlmann CH, Hutzschenreuter U, Overkamp F, Groschek M, Blumenstengel K, Pühse G, Steiner T (2011) Sequential therapies with sorafenib and sunitinib in advanced or metastatic renal cell carcinoma. World J Urol 29: 361–366
Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC European Association of Urology Guideline Group (2010) EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58: 398–406
Motzer RJ, Agarwal N, Beard C, Bhayani S, Bolger GB, Carducci MA, Chang SS, Choueiri TK, Hancock SL, Hudes GR, Jonasch E, Josephson D, Kuzel TM, Levine EG, Lin DW, Margolin KA, Michaelson MD, Olencki T, Pili R, Ratliff TW, Redman BG, Robertson CN, Ryan CJ, Sheinfeld J, Spiess PE, Wang J, Wilder RB (2011) NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw 9: 960–977
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20: 289–296
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124
Swedish National Board of Health and Welfare (2011) Quality and Efficiency in Swedish Cancer Care—Regional comparisons 2011. Available at http://www.socialstyrelsen.se/publikationer2012/2012-3-15
Porta C, Procopio G, Cartenì G, Sabbatini R, Bearz A, Chiappino I, Ruggeri EM, Re GL, Ricotta R, Zustovich F, Landi L, Calcagno A, Imarisio I, Verzoni E, Rizzo M, Paglino C, Guadalupi V, Bajetta E (2011) Sequential use of sorafenib and sunitinib in advanced renal-cell carcinoma (RCC): an Italian multicentre retrospective analysis of 189 patient cases. BJU Int 108 (8 Pt 2): E250–E257
Shek D, Tomlinson B, Brown M, Brunson A, Pan CX, Lara PN (2012) Epidemiological trends in renal cell carcinoma in the cytokine and post-cytokine eras: a registry analysis of 28,252 patients. Clin Genitourin Cancer 10: 93–98
Stadler WM, Figlin RA, McDermott DF, Dutcher JP, Knox JJ, Miller WH, Hainsworth JD, Henderson CA, George JR, Hajdenberg J, Kindwall-Keller TL, Ernstoff MS, Drabkin HA, Curti BD, Chu L, Ryan CW, Hotte SJ, Xia C, Cupit L, Bukowski RM ARCCS Study Investigators (2010) Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 116: 1272–1280
Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundström A, Westerholm B, Rosén M (2007) The new Swedish Prescribed Drug Register-opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16: 726–735
Woldrich JM, Mallin K, Ritchey J, Carroll PR, Kane CJ (2008) Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993–2004. J Urol 179: 1709–1713
Acknowledgements
This study was sponsored by Pfizer Inc. Medical writing support was provided by Andy Gannon at ACUMED (New York, NY, USA) with funding from Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
UH has reported receiving consultant/advisory fees from GlaxoSmithKline, Pfizer, Bayer, and Novartis, and research funding from Novartis. PS has reported receiving consultant/advisory fees from GlaxoSmithKline, Roche, Pfizer, and Novartis, honoraria from Bayer Schering Pharma, GlaxoSmithKline, Roche, and Pfizer, and research funding from Bayer Schering Pharma and Pfizer. SL has reported receiving honoraria from Roche, GlaxoSmithKline, and Pfizer, and research funding from Wyeth and Pfizer. BL has reported receiving consultant/advisory honoraria from Pfizer, Novartis, GlaxoSmithKline, and Bayer. TW, MJ, and RS are full-time employees of Pfizer. TW and RS hold Pfizer stock. JK is an employee of JK Biostatistics AB (Stockholm, Sweden) and was a paid contractor of Pfizer in the development of this manuscript and the analysis and interpretation of the data.
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wahlgren, T., Harmenberg, U., Sandström, P. et al. Treatment and overall survival in renal cell carcinoma: a Swedish population-based study (2000–2008). Br J Cancer 108, 1541–1549 (2013). https://doi.org/10.1038/bjc.2013.119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.119
Keywords
This article is cited by
-
Fibroblast growth factor receptor type 4 as a potential therapeutic target in clear cell renal cell carcinoma
BMC Cancer (2023)
-
Nephrectomy improves the survival of metastatic renal cell cancer patients with moderate to good performance status—results from a Finnish nation-wide population-based study from 2005 to 2010
World Journal of Surgical Oncology (2021)
-
Prognosis of Japanese metastatic renal cell carcinoma patients in the targeted therapy era
International Journal of Clinical Oncology (2021)
-
Changes in therapy and survival of metastatic renal cell carcinoma in Estonia
BMC Cancer (2020)
-
Treatment patterns and health outcomes in metastatic renal cell carcinoma patients treated with targeted systemic therapies in the UK
BMC Cancer (2020)