Abstract
Background:
No data are available on the pharmacogenetics of metronomic chemotherapy in prostate cancer. The aim of this study was to evaluate the association between VEGF-A sequence variants and prostate-specific antigen (PSA) progression, progression-free survival (PFS) and overall survival (OS), in advanced castration-resistant prostate cancer patients treated with metronomic cyclophosphamide (CTX), celecoxib and dexamethasone.
Methods:
Forty-three patients were enrolled, and genomic DNA was extracted. VEGF-A gene SNPs (−2578A/C, −634C/G, +936C/T) were analysed using TaqMan PCR assays. Hardy–Weinberg equilibrium was tested for each SNP, and genetic effects were evaluated by Fisher’s exact test. PFS and OS were analysed with GraphPad Prism software, using the product limit method of Kaplan and Meier, and comparing survival curves using both the log-rank test and the Gehan–Wilcoxon test. We used Bonferroni correction to account for multiple testing, and a two-tailed P-value of <0.017 was considered statistically significant.
Results:
Overall, 20 patients (46%) experienced a reduction in PSA levels from baseline and, among them, 14 (32%) showed a confirmed PSA ≥50% decrease. In non-responders, the −2578CC genotype was more frequent (18.60% vs 2.33% in responders; P=0.0212) whereas the −634CC genotype frequency was 22.73% vs 0% in responders (P=0.0485). With regard to PFS, patients harbouring the −634CC genotype had a median PFS of 2.2 months whereas patients with the genotype −634CG/GG had a median PFS of 6.25 months (P=0.0042).
Conclusion:
The −634CC genotype is significantly associated with a shorter PFS in patients treated with a metronomic CTX schedule.
Similar content being viewed by others
Main
Metronomic chemotherapy—the frequent administration of low doses of chemotherapeutic drugs (Pasquier et al, 2010)—has received remarkable interest from clinical oncologists over the last decade, particularly for its use in palliative care. The advantages of metronomic chemotherapy regimens include their low toxicity profile and low cost (Bocci et al, 2005), particularly when off patent drugs such as cyclophosphamide (CTX) are used (Penel et al, 2012). The antitumour effects of metronomic chemotherapy can be in part attributed to (i) the inhibition of angiogenesis and vasculogenesis (Kerbel and Kamen, 2004) and (ii) the blockade of circulating endothelial progenitor cells (Calleri et al, 2009). Furthermore, depending on the type of drug that is administered metronomically, direct cytotoxic effects on tumour cells and stimulation of the immune system also have been reported (Rozados et al, 2010). Metronomic oral CTX, as well as its combination with other drugs such as celecoxib (CXB) or dexamethasone (DEX), is a promising clinical approach to treat castration-resistant metastatic prostate cancer (Fontana et al, 2010a, 2010b; Nelius et al, 2011; Penel et al, 2012). In addition, the antitumour and antiangiogenic activity of metronomic CTX has been demonstrated in various experimental models of human prostate cancer (Man et al, 2002; Emmenegger et al, 2007, 2010, 2011).
The importance of the angiogenic process in prostate cancer progression, and the correlation between tumour blood vessel density and clinical (or preclinical) outcome have already been described in the literature (Hrouda et al, 2003; Nicholson and Theodorescu, 2004). Thus, there are reports demonstrating altered expression of angiogenic factors such as vascular endothelial growth factor (VEGF-A; Botelho et al, 2010), and inhibition of tumour growth after treatment with antiangiogenic therapies (Kluetz et al, 2010), including metronomic chemotherapy (Fontana et al, 2009; Nelius et al, 2010). Moreover, the clinical activity of metronomic CTX has been significantly associated with a decrease in plasma/serum VEGF-A levels (Calleri et al, 2009; Fontana et al, 2009). In the study by Fontana et al (2009), the VEGF levels markedly increased in non-responder prostate cancer patients after 20 days of metronomic treatment, and they remained significantly higher than in responders for >80 days. In contrast, VEGF concentrations in responder patients constantly decreased to values equivalent to half the baseline levels. Calleri et al (2009) described that in a cohort of 15 long-term responders to metronomic chemotherapy, the circulating VEGF-A levels measured after 2 months of therapy were significantly reduced. In contrast, in those same patients VEGF-A levels significantly increased at the time of disease progression. That observation suggests a possible role for VEGF-A in the mechanism of action of metronomic chemotherapy. In that respect, previous studies have shown that VEGF-A levels increase in patients whose tumours became resistant to metronomic chemotherapy (Allegrini et al, 2012). In addition, VEGF-A has been shown to be a potent survival factor for endothelial cells exposed to chemotherapeutic drugs (Tran et al, 2002). Taken together, these findings suggest that a (genetically determined) modulation of VEGF-A levels in the tumour microenvironment could impact the response of tumours to metronomic therapy. In that respect, it is noteworthy that VEGF-A gene polymorphisms, particularly those of its promoter, are responsible for variable production of the VEGF-A protein (Pasqualetti et al, 2007; Jain et al, 2009). Consequently, in this study we decided to test the hypothesis that VEGF-A functional polymorphisms could modulate the response of some prostate cancers to metronomic treatment. Our rationale was that patients with the VEGF-A –634CC genetic background may maintain higher tumour VEGF-A levels, which would contribute to a shorter progression-free survival (PFS) in such patients. Therefore, the study of VEGF-A polymorphisms could help identify those patients that are susceptible to, or resistant to, metronomic therapy, and (conceivably) to other antiangiogenic therapies (Vaziri et al, 2010; Bocci and Loupakis, 2011). It should also be noted that although metronomic chemotherapy has been in use for over a decade, it has not yet been studied from a pharmacogenetic perspective. Indeed, new molecular approaches to optimise metronomic chemotherapy protocols (and schedules) are urgently needed in order to improve the personalisation of this therapy for cancer patients.
The aim of this study was therefore to evaluate the possible association between VEGF-A gene polymorphisms and clinical efficacy in advanced castration-resistant prostate cancer patients treated with metronomic CTX (50 mg day–1), given in combination with CXB (400 mg day–1) and DEX (1 mg day–1).
Patients and methods
Study design and patients selection
This was an exploratory retrospective genetic study, with planned plasma sample collection from patients with castration-resistant advanced prostate cancer. These patients had been treated for at least 12 weeks with metronomic CTX (50 mg every day p.o.) plus CXB (200 mg twice a day p.o.) and DEX (1 mg once daily p.o.) at our institution. Forty-three patients were recruited, consecutively, at our institution, between January 2005 and December 2007. The Pisa University Ethics Committee approval was obtained to conduct this pilot study.
Eligibility criteria were as follows: histological diagnosis of prostate adenocarcinoma, castration-resistant disease, inability to receive standard chemotherapy because of toxicity concerns or failure of one or more previous chemotherapeutic lines of treatment for metastatic disease. All patients had measurable disease progression and/or rising prostate-specific antigen (PSA) serum level in two consecutive measurements at least 1-week apart and in accordance with the recommendations by the ‘Prostate Cancer Clinical Trials Working Group 2 (PCWG2)’ (Scher et al, 2008). Antiandrogen therapy (flutamide and bicalutamide) was discontinued at least 4–6 weeks before metronomic chemotherapy was started. The use of low-dose megestrol acetate for the amelioration of symptoms, and zoledronic acid, were allowed. All patients received luteinising hormone releasing hormone analogues.
Patients were monitored monthly, and assessed for treatment toxicity according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v.4.03, 2010; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf), and for treatment activity according to the recent ‘(PCWG2)’ guidelines (Scher et al, 2008).
VEGF-A genotyping and plasma VEGF-A detection at baseline
DNA extraction
Forty-three prostate cancer patients were enrolled in this study, and genomic DNA was available from the corresponding 43 blood samples. Blood samples (3 ml) were collected in EDTA tubes. DNA extraction was performed using QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA).
VEGF genotyping
Allelic discrimination of VEGF-A –2578A/C (rs699947), –634C/G (rs2010963) and 936C/T (rs3025039), was performed using an ABI PRISM 7900 SDS (Applied Biosystems, Carlsbad, CA, USA) and with validated TaqMan SNP genotyping assays (Applied Biosystems). PCR reactions were carried out according to the manufacturer’s protocol. Hardy–Weinberg equilibrium was tested for each SNP, using the following freeware software: PHASE and Arlequin version 3.1 (Stephens et al, 2001; Excoffier et al, 2005; Stephens and Scheet, 2005).
SNP selection
The three VEGF-A SNPs included in our study were selected on the basis of three main considerations: (i) thus far, in prostate cancer patients, no clear association has been reported between our chosen SNPs and risk or prognosis of prostate cancers (Jain et al, 2009); (ii) the three SNPs have been significantly associated with therapeutic efficacy of antiangiogenic drugs (e.g., bevacizumab) in other tumour types (Schneider et al, 2008; Etienne-Grimaldi et al, 2011); (iii) each SNP has been associated with modulation of VEGF-A expression, or with different plasma levels of VEGF-A.
Baseline VEGF-A levels
Baseline (i.e., before initiation of metronomic chemotherapy) VEGF-A levels could be determined for 28 patients for whom plasma samples were available to us, and these were analysed by ELISA for total concentration of human VEGF-A (Quantikine, cat. DVE00, R&D Systems, Minneapolis, MN, USA). The experimental procedures were carried out according to the ELISA kit protocol, which was standardised and validated by the manufacturer. The relative optical density was determined using a Multiskan Spectrum microplate reader (Thermo Labsystems, Milan, Italy) set to 450 nm, with wavelength correction set to 540 nm. The results were expressed as pg of VEGF-A per ml of plasma.
Statistical analyses
Progression-free survival and overall survival (OS), were calculated from the date of the first chemotherapy administration to the date of either disease progression or death, respectively. Progression-free survival and OS were analysed by the GraphPad Prism software (package version 5.0; GraphPad Software Inc., San Diego, CA, USA), using the product limit method of Kaplan and Meier, and comparing survival curves using both the log-rank test and the Gehan–Wilcoxon test. Fisher’s exact test was used to compare genotype frequencies between responders and non-responders. We defined as responders those patients who had a decrease in PSA of ≥50%, and a PSA stabilisation of ≥6 months. We used the Bonferroni correction to account for multiple testing, and a two-tailed P-value <0.017 (=0.05/3 SNPs) was considered statistically significant (Hsiao et al, 2007). Statistical analyses were performed using the GraphPad Prism software. The relationship between VEGF-A expression and the different genotypes was assessed using the non-parametric Kruskal–Wallis test (Loupakis et al, 2011).
Results
Characteristics of the patients
The patient characteristics relevant to this study are listed in Table 1. Forty-three consecutive patients were treated with CTX plus CXB and DEX continuously, for at least 12 weeks. The median age was 81 years (range 52–92). Nineteen patients (44%) had an ECOG (Eastern Cooperative Oncology Group) performance status of 0, and 24 (56%) had a status ≥1. The ECOG criteria are as follows: grade 0, the patient is fully active, able to carry on all pre-disease performance without restriction; grade 1, the patient is restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; grade 2, the patient is ambulatory and capable of all self-care but unable to carry out any work activities (Oken et al, 1982). The median baseline serum PSA level was 75.1 ng ml–1 (range 4.7–5000 ng ml–1). Bone and lymphnodes were the most frequent sites of metastasis (81% and 26%, respectively). Nine patients (21%) had undergone previous prostatectomy and six patients (14%) had received prior radiotherapy for their primary tumours. Furthermore, 25 patients (58%) received one or more chemotherapeutic regimens, including docetaxel-based (23 patients, 53%) and mitoxantrone-based (17 patients, 39%) chemotherapy. Zoledronic acid was administered to patients with bone metastases (i.e., 35 patients, 81%). Metronomic chemotherapy was administered for a median duration of 212 days (range 80–644 days). Five patients (11%) refused to receive CXB. The median follow-up was 30.7 months (95% CI 13.8–47.6 months).
It should be noted that 17 out of 43 patients (39%) received further treatments after disease progression; thus, 14 patients (32%) were treated with one or more chemotherapy regimens (eight docetaxel, six vinorelbine and two mitoxantrone), and 2 patients (4.6%) received further hormonal manipulations. Furthermore, one patient (2.4%) continued on the metronomic chemotherapy regimen, in accordance with the patient’s wishes and as a consequence of improvements in his symptoms and in his quality of life.
Toxicity
Metronomic CTX plus CXB and DEX were overall very well tolerated. Indeed, no grades 3–4 haematological or non-haematological toxicities were observed among the 43 patients. Seventeen patients (39.5%) experienced NCI-CTC grade 1 anaemia, and 12 patients (28%) experienced grade 1 asthenia. Seven patients (16.3%) developed a NCI-CTC grade 2 anaemia, and six patients (14%) developed grade 2 thrombocytopenia. Only one patient showed grade 2 neutropenia and anorexia (2.3%). No major cardiovascular events and no toxicity-related deaths were observed (Table 2).
Clinical activity
All patients received metronomic therapy for at least 12 weeks, and they were evaluated for their PSA response according to the recommendations of the PCWG2 (Scher et al, 2008). Figure 1 shows a waterfall plot, indicating the percentage change in PSA from baseline to 12 weeks into therapy for non-responding patients, or the maximal PSA decrease at any time point for those patients that responded to treatment.
Overall, 20 patients (46%) experienced a reduction in PSA levels from baseline (and the decrease ranged from 9% to 99%). Among these 20 patients, 14 (32%) showed a confirmed level of PSA ≥50% decrease, and 1 patient had stable PSA levels. Of note, among the 20 patients that showed a reduction of PSA, 11 had not received any prior chemotherapy. The remaining nine patients had previously been administered chemotherapy (median=2 regimens). Moreover, among the 23 patients that showed no reduction or even an increase in PSA levels, 16 had previously been treated with chemotherapy. The other seven patients did not receive any prior chemotherapy.
The median PFS was 5.26 months (range 0.39–24.33; 95% CI 2.42–7.32 months; Figure 2A) and the median OS was and 17.39 months (range 2.53–53.45 months; 95% CI 13.10–21.68 months, Figure 2B). Moreover, the median PFS of responders was 9.66 months (range 2.53–24.33 months; 95% CI 4.91–12.31 months), whereas the PFS of non-responders was 2.3 months (range 0.39–11.34 months; 95% CI 0.72–3.88 months). The median OS of responding patients was 22.26 months (range 2.53–53.45; 95% CI 13.9–29.42 months), whereas the median OS of non-responders was 12.66 (range 3.42–31.79; 95% CI 8.46–16.88 months).
Pharmacogenetic analyses
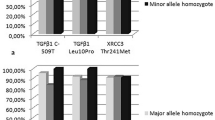
The allele frequencies for the VEGF-A gene polymorphisms in the 43 patients enrolled in our study are shown in Table 3. The distribution of genotypes in the 43 patients did not deviate from Hardy–Weinberg equilibrium (–2578C/A, P=0.053; –634G/C, P=0.180; +936 C/T, P=0.389).
In order to be able to interpret the pharmacogenetic data in relation to the biochemical responses, we defined as responders (to the metronomic schedule) those patients who had a decrease in PSA of ≥50%, and a PSA stabilisation of ≥6 months, as indicative of a long-term biochemical control of the disease. In non-responders, the –634CC VEGF-A genotype frequency was 22.73%, whereas no patient with CC genotype was observed in the responder’s group (P=0.0485). Indeed, in comparing responders and non-responders by a −2578CC vs −2578AC/AA genotype analysis, we demonstrated that the −2578CC genotype was more frequent (18.60% vs 2.33%) in non-responders, with a P-value of 0.0212 (although not significant by Bonferroni’s correction).
A relevant finding of our study was the identification of a VEGF-A genotype that was significantly associated with PFS. Indeed, with regard to PFS, patients harbouring the −634CC VEGF-A genotype had a median PFS of 2.2 months (95% CI 0.45–3.95 months) whereas patients with genotype –634CG/GG VEGF-A had a median PFS of 6.25 months (95% CI 3.28–8.62 months; P=0.0042; Figure 3). No significant differences were found in OS for the −634C/G genotypes. Nor did we find any significant differences and PFS and OS for the −2578A/C genotypes.
Interestingly, the only patient carrying the 936TT VEGF-A genotype had a PFS of 0.46 months and an OS of 3.94 months whereas the rest of the patient population with the genotype 936CT/CC VEGF-A had a median PFS of 5.26 months and a median OS of 17.98 months.
Plasma VEGF-A levels at baseline were not influenced by any of the studied VEGF-A SNPs; indeed, no significant relationship was observed between VEGF-A plasma concentrations and analysed SNPs from the promoter (−2578A/C), 5′-UTR (−634C/G), or from the 3′−UTR (+936C/T) region (Table 4).
Discussion
This study describes, for the first time, the pharmacogenetics of a metronomic chemotherapy regimen that consists of oral metronomic CTX regimen together with CXB and DEX, in advanced castration-resistant prostate cancer. In this regard, the individual genetic traits of patients may have a role in the response to chemotherapy or to antiangiogenic strategies, including to metronomic chemotherapy. However, there are currently no validated genetic or molecular biomarkers to predict or to monitor favourable clinical response or resistance to metronomic CTX (Penel et al, 2012). Therefore, efforts to identify such biomarkers are an important component of clinical oncology research. For example, our research has focused on the pharmacogenetics of tumour and germline SNPs of various genes in patients receiving antiangiogenic treatments such as metronomic chemotherapy (Pasqualetti et al, 2007). Here we report how we evaluated the impact of commonly reported VEGF-A sequence variants in relation to clinical outcomes following metronomic CTX. In particular, we decided to test the hypothesis that VEGF-A functional polymorphisms, such as the VEGF-A −634C/G, could modulate the response of some prostate cancers to metronomic treatment. Emphasis was placed on genetic susceptibilities in VEGF, because the clinical activity of metronomic CTX treatment has been significantly associated with a decrease in plasma/serum VEGF-A levels. One important finding of our study is the identification of a VEGF-A genotype (−634CC) that was associated with a shorter PFS among patients treated with metronomic chemotherapy. However, we were not able to demonstrate a relationship between this sequence variant and plasma VEGF levels.
The three investigated SNPs in our study have been defined functionally by different studies. In particular, Watson et al (2000) indicated that the –634C allele of VEGF-A increases VEGF-A production in peripheral blood mononuclear cells that are stimulated by lipopolysaccharide (Watson et al, 2000). Furthermore, Koukourakis et al (2004) investigated VEGF-A SNPs and their link with high VEGF-A expression in NSCLC specimens and normal lung. These authors found that low VEGF-A expression in tumour cells was significantly correlated with the presence of the −634GG VEGF-A genotype. In addition, patients with the −634GG VEGF-A genotype also had significantly lower tumour vascular density. Interestingly, VEGF-A production also has been found to be significantly higher in cells from −2578CC homozygotes than in those from −2578 AA individuals (Shahbazi et al, 2002), and patients harbouring the +936C allele had VEGF-A plasma levels significantly higher than non-carriers (Renner et al, 2000). Based on these published data, we hypothesise that patients with the −634CC or −2578CC genetic background may maintain higher tumour VEGF-A levels despite the administration of metronomic chemotherapy (Figure 4). In that respect, VEGF-A is known to act as a potent survival and chemoprotectant factor for endothelial cells exposed to chemotherapeutic drugs (Tran et al, 2002). Indeed, high concentrations of VEGF-A significantly reduced the pro-apoptotic potency of chemotherapy on both micro- and macrovascular endothelial cells with phosphatidylinositol 3-kinase-dependent, and survivin-dependent, mechanisms. For these reasons, a (genetically determined) modulation of VEGF-A in the tumour microenvironment could have a decisive role in the response of a tumour to metronomic therapy (Figure 4).
Despite the above-mentioned studies, large clinical trials involving prostate cancer patients did not find any correlation between VEGF-A baseline plasma levels and VEGF-A −2578C/A, −634G/C and +936C/T SNPs (Pander et al, 2007). Interestingly, our pilot study experience also appears to confirm these findings, because we found no significant association between any VEGF-A genotype and baseline VEGF plasma levels. These data seem to suggest that VEGF-A plasma levels may not be a good proxy for VEGF-A levels expressed in prostate cancer. In our opinion, this finding may be explained (for example) by biological conditions within the tumour microenvironment, such as VEGF-A upregulation because of hypoxia (Stewart et al, 2010) or to mutant H- or K-ras oncogenes (Rak et al, 1995) in cancer cells. Moreover, the experimental approaches used to measure VEGF-A plasma levels come with some known methodological pitfalls (e.g., the centrifugation procedures to obtain the plasma samples may cause platelet release of VEGF-A; Jelkmann, 2001) that may limit their extensive use in clinical trials. Thus, further studies in this area are greatly needed in order to clarify the phenotypes associated with specific VEGF-A SNPs in prostate cancer patients.
Although no published data are currently available on the role of VEGF-A SNPs in predicting the response to, and survival for, CTX metronomic chemotherapy for prostate cancer, a possible comparison (with some distinctions) could be made with a recent pilot study of Lenz’s group. In that study, 70 recurrent/metastatic ovarian cancer patients were treated with metronomic CTX and bevacizumab (Schultheis et al, 2008). Although patients with the VEGF-A +936CT genotype had a longer median PFS, compared with those with the TT genotype, these results were found to be not statistically significant (Schultheis et al, 2008). Moreover, the −634C/G SNP did not show any significant correlation with patient outcome. Instead, patients genotyped A/A or A/T for the IL-8 T251A gene polymorphism had a statistically significantly lower response rate than those with the genotype T/T (Schultheis et al, 2008).
This study recruited, consecutively, 43 patients treated with CTX metronomic chemotherapy at our institution between January 2005 and December 2007. An obvious limitation of this work is that the correlation between PFS and genotypes was assessed on a limited number of patients. A key point in pharmacogenetic studies is the low rate of ‘successful’ determinants that translate to the clinic. This is in contrast to the enthusiasm with which preliminary data are often received by the scientific community. Thus, as argued by us, initial data from pilot studies should be scrutinised with the most accurate statistical correction (Bocci and Loupakis, 2011), and efforts should be made to validate the preliminary data with randomised, prospective phase III clinical trials. For this reason, in this study we applied a strict Bonferroni’s correction to our data in order to avoid the risk of false-positive associations. Furthermore, we have already planned a prospective, randomised phase III clinical trial.
There are also some limitations with our findings in regards to the role of SNPs as pharmacogenetic markers, which could be also prognostic factors. Indeed, it is possible that some genotypes may be associated with a better (or worse) disease outcome, independently from the efficacy of the administered drug. This scenario would be of concern in single arm, retrospective, phase II studies where a comparison with a control arm (a different drug) is absent. However, the importance of pharmacogenetic pilot studies is that they may pave the way for new areas of research. Furthermore, they may guide the design of future, randomised, controlled and multi-institutional phase III studies (Taioli et al, 2011). In that respect, the collaborative efforts with investigators who spearhead prostate cancer consortia will be particularly important in providing a wider series of patients to validate our results. Thus, for example, in the planned phase III clinical trial we have included a full coverage of genes and genetic variants of the VEGF-A pathway (e.g., VEGFR-2, HIF-1α and HIF-2α). Moreover, the planned study will also include the analysis of SNPs of genes involved in the metabolism of CTX, such as CYP2B6, 3A4 and 2C9. The SNPs that could enhance or decrease the enzymatic activity of the above described CYPs may in turn alter the tumour response to metronomic chemotherapy, and could therefore have an impact on the survival of patients treated with such regimens.
In conclusion, our pilot study suggests that the −634CC genotype is significantly associated with a shorter PFS in patients treated with a metronomic CTX schedule. These findings represent a relevant effort to identify novel clinical markers for metronomic chemotherapy, which can be applied in future phase III clinical trials.
Change history
20 August 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Allegrini G, Di Desidero T, Barletta MT, Fioravanti A, Orlandi P, Canu B, Chericoni S, Loupakis F, Di Paolo A, Masi G, Fontana A, Lucchesi S, Arrighi G, Giusiani M, Ciarlo A, Brandi G, Danesi R, Kerbel RS, Falcone A, Bocci G (2012) Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers. Angiogenesis 15 (2): 275–286.
Bocci G, Loupakis F (2011) Bevacizumab pharmacogenetics in tumor treatment: still looking for the right pieces of the puzzle. Pharmacogenomics 12 (8): 1077–1080.
Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS, Del Tacca M (2005) Cyclophosphamide-methotrexate 'metronomic' chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol 16 (8): 1243–1252.
Botelho F, Pina F, Lunet N (2010) VEGF and prostatic cancer: a systematic review. Eur J Cancer Prev 19 (5): 385–392.
Calleri A, Bono A, Bagnardi V, Quarna J, Mancuso P, Rabascio C, Dellapasqua S, Campagnoli E, Shaked Y, Goldhirsch A, Colleoni M, Bertolini F (2009) Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res 15 (24): 7652–7657.
Emmenegger U, Francia G, Chow A, Shaked Y, Kouri A, Man S, Kerbel RS (2011) Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia 13 (1): 40–48.
Emmenegger U, Francia G, Shaked Y, Kerbel RS (2010) Metronomic chemotherapy: principles and lessons learned from applications in the treatment of metastatic prostate cancer. Recent Results Cancer Res 180: 165–183.
Emmenegger U, Shaked Y, Man S, Bocci G, Spasojevic I, Francia G, Kouri A, Coke R, Cruz-Munoz W, Ludeman SM, Colvin OM, Kerbel RS (2007) Pharmacodynamic and pharmacokinetic study of chronic low-dose metronomic cyclophosphamide therapy in mice. Mol Cancer Ther 6 (8): 2280–2289.
Etienne-Grimaldi MC, Formento P, Degeorges A, Pierga JY, Delva R, Pivot X, Dalenc F, Espie M, Veyret C, Formento JL, Francoual M, Piutti M, de Cremoux P, Milano G (2011) Prospective analysis of the impact of VEGF-A gene polymorphisms on the pharmacodynamics of bevacizumab-based therapy in metastatic breast cancer patients. Br J Clin Pharmacol 71 (6): 921–928.
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1: 47–50.
Fontana A, Bocci G, Galli L, D'Arcangelo M, Derosa L, Fioravanti A, Orlandi P, Barletta MT, Landi L, Bursi S, Minuti G, Bona E, Grazzini I, Danesi R, Falcone A (2010a) Metronomic cyclophosphamide in elderly patients with advanced, castration-resistant prostate cancer. J Am Geriatr Soc 58 (5): 986–988.
Fontana A, Falcone A, Derosa L, Di Desidero T, Danesi R, Bocci G (2010b) Metronomic chemotherapy for metastatic prostate cancer: a 'young' concept for old patients? Drugs Aging 27 (9): 689–696.
Fontana A, Galli L, Fioravanti A, Orlandi P, Galli C, Landi L, Bursi S, Allegrini G, Fontana E, Di Marsico R, Antonuzzo A, D’Arcangelo M, Danesi R, Del Tacca M, Falcone A, Bocci G (2009) Clinical and pharmacodynamic evaluation of metronomic cyclophosphamide, celecoxib, and dexamethasone in advanced hormone-refractory prostate cancer. Clin Cancer Res 15 (15): 4954–4962.
Hrouda D, Nicol DL, Gardiner RA (2003) The role of angiogenesis in prostate development and the pathogenesis of prostate cancer. Urol Res 30 (6): 347–355.
Hsiao PJ, Lu MY, Chiang FY, Shin SJ, Tai YD, Juo SH (2007) Vascular endothelial growth factor gene polymorphisms in thyroid cancer. J Endocrinol 195 (2): 265–270.
Jain L, Vargo CA, Danesi R, Sissung TM, Price DK, Venzon D, Venitz J, Figg WD (2009) The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol Cancer Ther 8 (9): 2496–2508.
Jelkmann W (2001) Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem 47 (4): 617–623.
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4 (6): 423–436.
Kluetz PG, Figg WD, Dahut WL (2010) Angiogenesis inhibitors in the treatment of prostate cancer. Expert Opin Pharmacother 11 (2): 233–247.
Koukourakis MI, Papazoglou D, Giatromanolaki A, Bougioukas G, Maltezos E, Sivridis E (2004) VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 46 (3): 293–298.
Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, Di Desidero T, Canu B, Schirripa M, Frumento P, Di Paolo A, Danesi R, Falcone A, Bocci G (2011) Pharmacodynamic and pharmacogenetic angiogenesis-related markers of first-line FOLFOXIRI plus bevacizumab schedule in metastatic colorectal cancer. Br J Cancer 104 (8): 1262–1269.
Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS (2002) Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res 62 (10): 2731–2735.
Nelius T, Klatte T, de Riese W, Haynes A, Filleur S (2010) Clinical outcome of patients with docetaxel-resistant hormone-refractory prostate cancer treated with second-line cyclophosphamide-based metronomic chemotherapy. Med Oncol 27 (2): 363–367.
Nelius T, Rinard K, Filleur S (2011) Oral/metronomic cyclophosphamide-based chemotherapy as option for patients with castration-refractory prostate cancer: review of the literature. Cancer Treat Rev 37 (6): 444–455.
Nicholson B, Theodorescu D (2004) Angiogenesis and prostate cancer tumor growth. J Cell Biochem 91 (1): 125–150.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5 (6): 649–655.
Pander J, Gelderblom H, Guchelaar HJ (2007) Pharmacogenetics of EGFR and VEGF inhibition. Drug Discov Today 12 (23-24): 1054–1060.
Pasqualetti G, Danesi R, Del Tacca M, Bocci G (2007) Vascular endothelial growth factor pharmacogenetics: a new perspective for anti-angiogenic therapy. Pharmacogenomics 8 (1): 49–66.
Pasquier E, Kavallaris M, Andre N (2010) Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 7 (8): 455–465.
Penel N, Adenis A, Bocci G (2012) Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol 82 (1): 40–50.
Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS (1995) Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev 14 (4): 263–277.
Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E (2000) A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res 37 (6): 443–448.
Rozados VR, Mainetti LE, Rico MJ, Zacarias Fluck MF, Matar P, Scharovsky OG (2010) The immune response and the therapeutic effect of metronomic chemotherapy with cyclophosphamide. Oncol Res 18 (11-12): 601–605.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26 (7): 1148–1159.
Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD (2008) Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26 (28): 4672–4678.
Schultheis AM, Lurje G, Rhodes KE, Zhang W, Yang D, Garcia AA, Morgan R, Gandara D, Scudder S, Oza A, Hirte H, Fleming G, Roman L, Lenz HJ (2008) Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res 14 (22): 7554–7563.
Shahbazi M, Fryer AA, Pravica V, Brogan IJ, Ramsay HM, Hutchinson IV, Harden PN (2002) Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol 13 (1): 260–264.
Stephens M, Scheet P (2005) Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet 76 (3): 449–462.
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68 (4): 978–989.
Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC (2010) The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int 105 (1): 8–13.
Taioli E, Flores-Obando RE, Agalliu I, Blanchet P, Bunker CH, Ferrell RE, Jackson M, Kidd LC, Kolb S, Lavender NA, McFarlane-Anderson N, Morrison SS, Multigner L, Ostrande EA, Park JY, Patrick AL, Rebbeck TR, Romana M, Stanford JL, Ukoli F, Vancleave TT, Zeigler-Johnson CM, Mutetwa B, Ragin C (2011) Multi-institutional prostate cancer study of genetic susceptibility in populations of African descent. Carcinogenesis 32 (9): 1361–1365.
Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS (2002) A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA 99 (7): 4349–4354.
Vaziri SA, Kim J, Ganapathi MK, Ganapathi R (2010) Vascular endothelial growth factor polymorphisms: role in response and toxicity of tyrosine kinase inhibitors. Curr Oncol Rep 12 (2): 102–108.
Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE (2000) Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 12 (8): 1232–1235.
Acknowledgements
The present work was supported, in part, by AIRC (Italian Association for Cancer Research). We thank Professor Franco Bocci, Paloma Valenzuela and Eduardo Ramirez, for their assistance with editing the manuscript. Giulio Francia was in part supported by URI and IDR2 grants from the University of Texas at El Paso.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Orlandi, P., Fontana, A., Fioravanti, A. et al. VEGF-A polymorphisms predict progression-free survival among advanced castration-resistant prostate cancer patients treated with metronomic cyclophosphamide. Br J Cancer 109, 957–964 (2013). https://doi.org/10.1038/bjc.2013.398
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.398
Keywords
This article is cited by
-
Pharmacodynamic biomarkers in metronomic chemotherapy: multiplex cytokine measurements in gastrointestinal cancer patients
Clinical and Experimental Medicine (2021)
-
Impact of CTLA-4 blockade in conjunction with metronomic chemotherapy on preclinical breast cancer growth
British Journal of Cancer (2017)
-
Current achievements and future perspectives of metronomic chemotherapy
Investigational New Drugs (2017)
-
Metronomic cyclophosphamide therapy in hormone-naive patients with non-metastatic biochemical recurrent prostate cancer: a phase II trial
Medical Oncology (2016)
-
Interplay between 3′-UTR polymorphisms in the vascular endothelial growth factor (VEGF) gene and metabolic syndrome in determining the risk of colorectal cancer in Koreans
BMC Cancer (2014)