Abstract
Background:
Annexin A2 (AnxA2), a calcium-dependent phospholipid binding protein, is abundantly present at the surface of triple-negative and Herceptin-resistant breast cancer cells. Interactions between cell-surface AnxA2 and tyrosine kinase receptors have an important role in the tumour microenvironment and act together to enhance tumour growth. The mechanism supporting this role is still unknown.
Methods:
The membrane function of AnxA2 was blocked by incubating cells with anti-AnxA2 antibodies. Western blotting, immunoprecipitation, immunofluorescence, 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT), flow cytometry, Clonogenic, and wound-healing assays were performed in this study.
Results:
We demonstrate that AnxA2 interacts with epidermal growth factor receptor (EGFR) at the cell surface and has an important role in cancer cell proliferation and migration by modulating EGFR functions. Blocking AnxA2 function at the cell surface by anti-AnxA2 antibody suppressed the EGF-induced EGFR tyrosine phosphorylation and internalisation by blocking its homodimerisation. Furthermore, addition of AnxA2 antibody significantly inhibited the EGFR-dependent PI3K-AKT and Raf-MEK-ERK downstream pathways under both EGF-induced and basal growth conditions, resulting in lower cell proliferation and migration.
Conclusions:
These findings suggest that cell-surface AnxA2 has an important regulatory role in EGFR-mediated oncogenic processes by keeping EGFR signalling events in an activated state. Therefore, AnxA2 could potentially be used as a therapeutic target in triple-negative and Herceptin-resistant breast cancers.
Similar content being viewed by others
Main
Breast cancer is the second leading cause of cancer-related death among women in the United States of America. On the basis of gene expression profiles, breast cancer can be classified into four major subgroups: luminal A and luminal B types that express the oestrogen receptor (ER) and progesterone receptor (PR), those that overexpress the human epidermal growth factor receptor 2 (ErbB2/HER2), and basal-like cancers that express cytokeratins 5/6, 14, 17 and epidermal growth factor receptor (EGFR/ErbB1/HER1) (Perou et al, 2000; Sørlie et al, 2001). Approximately 15–20% of basal-like cancer cases typically have low expression of ER, PR, and HER2 and are commonly referred to as triple-negative breast cancer (TNBC) (Yehiely et al, 2006; Stevens et al, 2013). Triple-negative breast cancer are difficult to treat, as they do not respond to hormone and Herceptin therapies and are especially aggressive due to their frequent recurrence and high metastatic potential (Dent et al, 2007; Hurvitz and Finn, 2009). In ER+, PR+, and HER2+ breast cancers, alternative survival pathways are commonly seen to be upregulated upon inhibition of these receptors, and acquire resistance to such therapies over time (Pohlmann et al, 2009; Osborne and Schiff, 2011). Therefore, the identification of common molecular markers in triple-negative and Herceptin-resistant breast cancers is necessary to enable the design of effective targeted therapies.
Annexin A2 (AnxA2), a calcium-dependent phospholipid-binding protein, exists as a heterotetrameric complex with the plasminogen receptor protein, S100A10 (p11), at the cell surface and has a key role in several membrane-related events, including fibrinolysis, exocytosis and endocytosis, cell-cell adhesion, ion channel conductance, and membrane-cytoskeletal interactions (Ling et al, 2004; Flood and Hajjar, 2011; Valapala and Vishwanatha, 2011; Bharadwaj et al, 2013). It is aberrantly overexpressed in patients with both invasive ductal mammary carcinoma and ductal carcinoma in situ (DCIS). In contrast, it is undetectable in normal and hyperplastic ductal epithelial cells and ductal complexes, suggesting a pivotal role of AnxA2 in breast tumour malignancy and invasiveness (Sharma et al, 2006). While the expression of AnxA2 increases with the aggressiveness of breast cancer (Sharma et al, 2006; Shetty et al, 2012), the surface expression of AnxA2 increases with acute or chronic treatment of Herceptin immunotherapy and chemotherapeutic drugs such as anthracyclines and taxanes (Chuthapisith et al, 2007; Zhang et al, 2009; Shetty et al, 2012). Previous studies indicate that AnxA2 is a potential therapeutic target to inhibit neoangiogenesis and its dependent tumour growth and metastasis in patients with breast cancer and other malignant diseases (Ling et al, 2004; Zhang et al, 2009; Kesavan et al, 2010; Valapala et al, 2011a). Treatment with angiostatin inhibits breast tumour growth and lung metastasis formation via its binding to AnxA2, thus inhibiting AnxA2-mediated plasmin generation (Tuszynski et al, 2002; Sharma and Sharma, 2007). The administration of anti-AnxA2 antibody potentially inhibits the growth of human breast tumour in a xenograft model, suggesting a relevant role of AnxA2 in tumour progression (Sharma et al, 2012). This effect is associated with a marked reduction in cell proliferation as well as angiogenesis in breast tumour. However, the molecular mechanisms governing the proliferative response to AnxA2 in breast tumour remain to be elucidated.
The cell-surface AnxA2 levels were higher in metastatic breast cancer cells than in non-metastatic cells. However, little is known about the implications of this for breast cancer cell proliferation and migration. In our previous studies, we have demonstrated that treatment with Herceptin leads to overexpression and activation of AnxA2 and EGFR, which further contributes to oncogenesis and Herceptin resistance (Shetty et al, 2012). In addition, we found an inverse correlation of HER2 and AnxA2 expression in various breast cancer tumours and cell lines. We further extended our studies to triple-negative and Herceptin-resistant breast cancer phenotype, where EGFR expression is upregulated, and validated the importance of AnxA2 in keeping EGFR signalling in an activated state, leading to cancer cell proliferation and migration. The present studies elucidate the role of cell-surface AnxA2 in the regulation of EGFR signalling, and determine whether AnxA2 antibody can inhibit the cell proliferation and migration of triple-negative and Herceptin-resistant breast cancer cells. These studies report a functional association of AnxA2 with EGFR at the cell surface of triple-negative and Herceptin-resistant breast cancer cells. Further, we demonstrate that EGF-induced EGFR dimerisation, and tyrosine phosphorylation and its internalisation, were inhibited with the pretreatment of AnxA2 antibody.
Materials and methods
Cell lines
MCF-7, T47D, MCF-10A, HCC-70, SK-BR-3, and MDA-MB-231 breast cancer cell lines were purchased from the American Type Culture Collection (ATCC) and cultured according to ATCC recommendations. Herceptin-resistant breast cancer cell line JIMT-1 was procured from German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany).
Materials
DMEM, DMEM/F12, epidermal growth factor (EGF), and fetal bovine serum were purchased from GIBCO (Invitrogen, Carlsbad, CA, USA). Antibodies against EGFR, Annexin A2 (H-50), early endosome antigen 1 (EEA1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), whereas p-EGFR (Tyr-845), p-EGFR (Tyr-1045), p-EGFR (Tyr-1068), phospho-phosphatidylinositol 3-kinase (p-PI3K) p85 (Tyr-458)/p55 (Tyr-199), phospho-Phosphoinositide-dependent kinase-1 (p-PDK1) (Ser-241), p-AKT (Ser-473), AKT, and p-c-Raf (Ser-338) were from Cell Signaling Technology (Danvers, MA, USA). Annexin A2 (clone 5), phospho-Mitogen-activated protein kinase kinase 1/2 (p-MEK1/2) (Ser-218/Ser-222), extracellular signal-regulated protein kinase 1 (ERK1), and p-ERK1/2 (Thr-202/Tyr-204) antibodies were purchased from BD Pharmingen (BD Biosciences, San Jose, CA, USA). LY294002 was purchased from Cayman Chemical (Ann Arbor, MI, USA). Mouse anti-Annexin A2 (D1/274.5) antibody was a kind gift from Dr Tony Hunter, Salk Institute for Biological Studies, La Jolla, CA, USA. All other reagents and chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). Recombinant c-terminal His-tag EGFR (1–645 amino-acid residues) was purchased from OriGene Technologies (Rockville, MD, USA). ON-TARGET plus non-targeting small-interfering RNA (siRNA), ON-TARGET plus SMART pool EGFR, and tPA siRNA were purchased from GE Healthcare (Dharmacon, Lafayette, CO, USA).
EGFR dimerisation
Cells were grown to 80% confluence in 6-well tissue culture plates. After overnight serum starvation, cells were incubated with heat inactivated (D1/274.5) AnxA2 antibody or AnxA2 antibody (2 μg ml−1) for 2 h followed by EGF (50 ng ml−1) treatment for 20 min. Cells were then washed twice with ice-cold PBS and incubated with PBS containing sulfo-EGS (Ethylene Glycol bis (Sulfosuccinimidylsuccinate)) (5 mM) on ice for 2 h to crosslink EGFR. The crosslinking reaction was terminated by adding Tris (10 mM), followed by washing with PBS, and were lysed in RIPA buffer. The dimerisation of EGFR was detected by western blotting.
Clonogenic assay
In all, 500 cells were plated in 60 mm tissue culture dishes in full serum medium and allowed to adhere. After 12 h of serum starvation, cells were treated with heat inactivated AnxA2 (D1/274.5) antibody or AnxA2 (D1/274.5) antibody (2 μg ml−1) and incubated for an additional 2 h. After antibody incubation, one set of plates was treated with EGF (50 ng ml−1) and a second set of plates was treated with PBS only and incubated for 4 days. Colonies were washed with PBS, fixed with 4% paraformaldehyde, stained with crystal violet and quantified.
Immunofluorescence studies
Cells were grown to 50% confluence on glass coverslips in 12-well plates. The cells were exposed to EGF or FITC labelled EGF (50 ng ml−1). Untreated cells remained as controls. Treated and untreated cells were washed twice with ice-cold PBS, fixed with 4% paraformaldehyde for 30 min, and then permeabilised with 0.1% Triton X-100 for 20 min if required. The slides were then washed with PBS, incubated with 5% goat serum in PBS for 2 h, and then incubated with anti-AnxA2, anti-EEA1, or anti-EGFR antibodies that were diluted 1 : 100 in PBS overnight at 4 °C. After washing with ice-cold PBS three times, the coverslips were incubated with Alexa Fluor 488 goat anti-mouse IgG or Alexa Fluor 594 goat anti-rabbit IgG (Life Technologies, Eugene, OR, USA) that was diluted 1 : 400 in PBS for 2 h at room temperature in darkness. The coverslips were then washed with ice-cold PBS and mounted on glass slides with ProLong Gold anti-fade reagent containing DAPI (1.5 μg ml−1) (Invitrogen Inc., Eugene, OR, USA). The slides were examined using LSM 510 Meta confocal system equipped with an inverted microscope (Axio Observer Z1, Carl Zeiss, Thornwood, NY, USA).
Flow cytometry, immunoprecipitation, western blotting, RNA interference, wound-healing, and cell viability assays
Flow cytometry, immunoprecipitation, western blotting, wound-healing, and cell viability assays were performed as described earlier (Shetty et al, 2012)
Statistical analysis
Data are expressed as the mean±s.e. for each group. Statistical significance was determined by Student’s t-test and was set at P<0.05.
Results
Annexin A2 is overexpressed at the surface of triple-negative and Herceptin-resistant breast cancer cells and interacts with EGFR
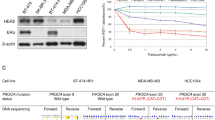
In our previous studies, we have shown that expression of AnxA2 is inversely correlated with the expression of HER2 in breast cancer clinical samples, as well as in cell lines (Shetty et al, 2012). Furthermore, siRNA-mediated downregulation of HER2 or Herceptin-mediated inhibition of HER2 function in HER2+ cells induces AnxA2 expression and its membrane translocation. In the present study, we have examined whether increased expression of AnxA2 in triple-negative and Herceptin-resistant breast cancer cells mobilises it to the outer membrane. First, we compared the cell-surface localisation of AnxA2 in triple-negative and Herceptin-resistant breast cancer cell lines with non-tumourigenic epithelial cells and ER+, PR+, and HER2+ breast cancer cell lines. We used Versene (0.53 mM EDTA in PBS) to wash the cells. Because AnxA2 heterotetramers are Ca2+-dependent phospholipid-binding proteins, the heterotetramer proteins should be stripped from the cell surface by Versene. We collected both total cell lysate and Versene-wash fractions and analysed them with western blotting. The blots presented in Figure 1A show that in triple-negative, as well as in Herceptin-resistant breast cancer cell lines, a large amount of AnxA2 is present in the membrane wash fraction. In ER+, PR+, and HER2+ cell lines, because of low expression, a minimal amount of AnxA2 is present at the cell surface. In non-tumourigenic epithelial cells, even though the expression of cellular AnxA2 is comparable to triple-negative and Herceptin-resistant breast cancer cells, membrane localisation is very low. These results suggest that in triple-negative and Herceptin-resistant breast cancer cells a large amount of AnxA2 is specifically translocated to the outer membrane domain.
AnxA2 expression in triple-negative and Herceptin-resistant breast cancer cell lines and its association with EGFR at cell surface. (A) Endogenous and cell surface AnxA2 protein was analysed by western blotting in a breast cancer cell line panel including non-tumourigenic epithelial cell line MCF-10A; ER expressing MCF7; HER-2 amplified SK-BR-3; ER and PR expressing T47D; triple-negative MDA-MB-231, HCC-70 and Herceptin-resistant JIMT-1. Cells were incubated with Versene for 10 min and then supernatant was collected by centrifugation for western blot analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control for total cell lysate. (B) MDA MB-231 and JIMT-1 cells were serum starved for 12 h, then treated with or without EGF (50 ng ml−1) for 20 min. Membrane/cytosolic fractions and immunoprecipitation were performed and analysed by western blotting. The purification of the membrane and cytosolic fractions were analysed by blotting with Na+/K+ ATPase and GAPDH antibody, respectively. Results are representative of two independent experiments. (C) MDA-MB-231 and JIMT-1 cells were stimulated with or without EGF (50 ng ml−1) for 20 min after 12 h serum starvation. The cells were fixed, blocked, and incubated with mouse or rabbit anti-AnxA2 and rabbit C-terminal anti-EGFR or mouse N-terminal anti-EGFR antibody without permeabilisation, followed by anti-mouse or rabbit Alexa 488 and anti-rabbit or mouse Alexa 595 secondary antibody. The slides were examined using LSM 510 Meta confocal microscope system. Yellow dots represent the colocalisation of green and red colors, which indicates that AnxA2 is colocalised with EGFR. The percentage of colocalisation was calculated analyzing a minimum of 10 cells for each treatment randomly taken from three independent experiments. Each bar represents the mean±s.e. (**P<0.01 vs control). (D) After 72 h of control and tPA siRNA transfection, JIMT-1 cells were lysed (lysis buffer: 10 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol and 1% CHAPS {3-[(3-Cholamidopropyl)-dimethylammonio]-1- propanesulfonate}) in the presence of a protease inhibitor mixture (EMD Millipore) and sonicated. The recombinant C-terminal His-tagged EGFR (1–645 amino acids) protein (2.0 μg) was incubated at 37 °C for 2 h with lysates from JIMT-1 cells transfected with control and tPA siRNA and then incubated with TALON superflow metal affinity resin (Clontech, Mountain View, CA). Beads were washed four times with lysis buffer supplemented with 5 mM imidazole and resuspended in SDS-PAGE sample buffer followed by western blot analysis.
Epidermal growth factor receptor and AnxA2 expression at the cell surface is required for tPA-induced activation of ERK1/2 that leads to cell proliferation in pancreatic cancer cells (Ortiz-Zapater et al, 2007). It is previously known that EGFR is localised in the lipid raft domain, which brings several signalling proteins together and activates the downstream signalling upon ligand stimulation (Yarden and Sliwkowski, 2001; Normanno et al, 2006; Lemmon and Schlessinger, 2010). Consistent with this, our previous finding also suggests the association of AnxA2 and EGFR in the membrane complex and knock-down of AnxA2 inhibits EGF-induced EGFR-mediated downstream signalling (Shetty et al, 2012). Considering the multiple functions of AnxA2 in tyrosine receptor signalling complexes, we have further performed immunoprecipitation experiments with membrane and cytosolic extracts of both MDA-MB-231 (triple-negative) and JIMT-1 (Herceptin-resistant) breast cancer cell lines, with or without EGF treatment. The results of western blot analysis showed that AnxA2 binds with EGFR in the membrane domain in both cell types (Figure 1B). When the immunoprecipitates obtained using EGFR antibodies were probed with AnxA2 antibody, the results (Figure 1B) further confirmed binding of EGFR with AnxA2. Together, the results of these experiments indicated that AnxA2 interacts with EGFR in both cell types. Interestingly, this interaction was independent of EGF stimulation, which suggests that AnxA2 is inherently present in the EGFR protein complex. To confirm whether this interaction occurs at extracellular or intracellular, we performed immunofluorescence studies in MDA-MB-231 and JIMT-1 cell lines using C-terminal and N-terminal specific anti-EGFR antibody without permeabilising the cells in the presence or absence of EGF (Figure 1C). After confocal microscopic analysis, we found that N-terminal antibody shows the colocalisation of EGFR with AnxA2 (yellow dots) which suggests that extracellular AnxA2 binds to extracellular domain of EGFR (Figure 1C). However, we could not observe any signal (red fluorescence) with C-terminal anti-EGFR antibody which suggests that it does not cross the cell membrane. After quantitative analysis, we found 7.6% and 14.2% increased colocalisation of AnxA2 with EGFR at the cell surface upon EGF stimulation in MDA-MB-231 and JIMT-1 cell lines, respectively (Figure 1C). Previously, it has been shown in pancreatic cancer cells that AnxA2 interacts with tissue plasminogen activator (tPA) and tPA interacts with EGFR (Ortiz-Zapater et al, 2007). To rule out the possibility that tPA is not a bridging protein between AnxA2 and EGFR, we performed the pull-down assay using purified C-terminal His-tag EGFR (1–645 amino acids) protein. In this assay, we used equal amount of lysates of control and tPA-depleted JIMT-1 cells (Supplementary Figure S1A) and incubated with His-tagged EGFR protein. The results of the western blot analysis showed that extracellular domain of EGFR binds with AnxA2 of the cell lysates prepared from control and tPA siRNA-transfected JIMT-1 cells (Figure 1D). To analyse further these direct interactions, we performed the colocalisation of AnxA2 with EGFR by immunofluorescence microscopy in tPA-depleted JIMT-1 cells. Our results showed that AnxA2 interacts with EGFR at the cell surface of JIMT-1 cells transfected with control or tPA siRNA (Supplementary Figure S1B). After quantitative analysis, we found 13.9% and 15.0% increased colocalisation of AnxA2 with EGFR at the cell surface upon EGF stimulation in control and tPA siRNA-transfected JIMT-1 cells, respectively (Supplementary Figure S1B).
AnxA2 antibody inhibits EGF-stimulated cell proliferation and migration
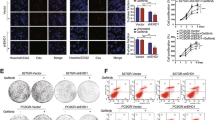
Annexin A2 and EGFR are the most frequently upregulated proteins in triple-negative and Herceptin-resistant breast cancer and have also been shown to correlate with tumour grade, stage, and metastasis (Shetty et al, 2012). Additionally, the knock-down of AnxA2 inhibits the EGF-induced EGFR phosphorylation and downstream signalling in MDA-MB-231 cells (Shetty et al, 2012). In our earlier results, we observed that AnxA2 at the cell surface interacts with extracellular domain of EGFR. Therefore, we questioned whether inhibition of cell-surface AnxA2 function by AnxA2 antibody can suppress the EGF-stimulated cell proliferation and migration. Inhibition of EGF-stimulated cell proliferation by AnxA2 antibody was evaluated by MTT and clonogenic assays. Here, heat inactivated AnxA2 (D1/274.5) antibody was used as an immunoglobulin control and their epitope recognition property towards AnxA2 protein was destroyed by heating. As shown in Figure 2A–D, the EGF-induced cell proliferation of MDA-MB-231 and JIMT-1 cells was inhibited in the presence of AnxA2 (D1/274.5) antibody as compared with the control or with treatment with heat inactivated AnxA2 (D1/274.5) antibody. The results of MTT assay showed that preincubation of cells with AnxA2 (D1/274.5) antibody in serum-free medium significantly decreased EGF-induced MDA-MB-231 (Figure 2A) and JIMT-1 (Figure 2B) cell proliferation by 22% and 30%, respectively. However, no difference in cell proliferation was observed in both cell types after AnxA2 (D1/274.5) antibody incubation in the absence of EGF. The inhibition of EGF-induced cell proliferation by AnxA2 antibody was further confirmed by clonogenic assay, where MDA-MB-231 (Figure 2C) and JIMT-1 cells (Figure 2D) were preincubated with AnxA2 antibody in a serum-free medium that was supplemented, or not, with EGF. Although EGF strongly promoted cell proliferation of MDA-MB-231 and JIMT-1 cells, this effect was significantly reduced by 130% and 73%, respectively, after AnxA2 antibody treatment. To determine whether AnxA2 antibody inhibits EGF-induced cell proliferation via EGFR, we further performed the MTT assay in JIMT-1 cells with EGFR knockdown. EGF-induced cell proliferation was completely abolished in EGFR-depleted JIMT-1 cells (Figure 2E). In addition, preincubation of cells with AnxA2 (D1/274.5) antibody did not affect the EGF-induced cell proliferation of EGFR-depleted JIMT-1 cells compared with control siRNA-transfected cells (Figure 2E). Together, these results further suggest that AnxA2 antibody inhibits the EGF-induced cell proliferation of MDA-MB-231 and JIMT-1 cells via EGFR.
AnxA2 antibody inhibits the EGF-induced cell proliferation of MDA-MB-231 and JIMT-1 cells. MDA-MB-231 (A) and JIMT-1 (B) cells (1 × 103) were plated in 96-well plates in a full serum medium. Next day, cells were serum starved for 12 h and then incubated with heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 (D1/274.5) antibody (2 μg ml−1) for 2 h, followed by EGF (50 ng ml−1) treatment. After 4 days of incubation, MTT assay was performed. Results presented are the percentage of cell proliferation in treated groups with respect to control cells (mean±s.e. (error bars); n=8). For clonogenic survival assay, MDA-MB-231 (C) and JIMT-1 (D) cells (500 cells per well) were seeded in a 60 mm tissue culture dish and allowed to adhere overnight. After 12 h of serum starvation, cells were incubated with heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 (D1/274.5) antibody (2 μg ml−1) for 2 h followed by EGF (50 ng ml−1) treatment and incubated for 4 days. After paraformaldehyde fixation and staining with crystal violet (0.05% in PBS), cells were photographed and quantified. Each bar represents the mean±s.e. of three independent experiments. (E) Depletion of EGFR was performed by the ON-TARGET plus SMART pool EGFR siRNA as per the manufacturer’s instructions (Thermo Scientific Dharmacon), and control JIMT-1 cells were treated with ON-TARGET plus non-targeting siRNA in a similar way. After 48 h of transfection, the cells were harvested and the expression of EGFR was examined by western blot analysis. After 24 h of control and EGFR siRNA transfection, 1 × 103 JIMT-1 cells per well were plated in 96-well plates in a full serum medium. Next day, cells were serum starved for 12 h and then incubated with heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 (D1/274.5) antibody (2 μg ml−1) for 2 h, followed by EGF (50 ng ml−1) treatment. After 4 days of incubation, MTT assay was performed as described previously (mean±s.e. (error bars); n=8). Each bar represents the mean±s.e. of three independent experiments. (*P<0.05; **P<0.01 vs control or heat inactivated AnxA2 antibody treatment group).
We have previously reported that knockdown of AnxA2 inhibits the cell motility and wound closure in metastatic breast cancer cells (Shetty et al, 2012). To further identify whether blocking cell-surface AnxA2 functions by AnxA2 antibody can inhibit the EGF-induced cell migration in triple-negative and Herceptin-resistant breast cancer cells, we performed an in vitro scratch wound-resealing assay. After time-lapse imaging, we observed that AnxA2 (D1/274.5) antibody preincubation resulted in 15% and 22% delay in wound closure after 24 h of wound formation in MDA-MB-231 (Figure 3A) and JIMT-1 (Figure 3B) cells, respectively, as compared with the control and with treatment with heat inactivated AnxA2 (D1/274.5) antibody. However, no difference in wound closure was observed in the absence of EGF with AnxA2 (D1/274.5) antibody pretreatment in both cell types. To assess further the role of EGFR in inhibition of EGF-induced cell migration by AnxA2 antibody, we performed an in vitro wound-resealing assay in EGFR-depleted JIMT-1 cells. As shown in Figure 3C, EGF-induced cell migration was completely abolished in EGFR-depleted JIMT-1 cells. In addition to this, preincubation of cells with AnxA2 (D1/274.5) antibody did not affect the EGF-induced wound closer after 24 h of wound formation in EGFR-depleted JIMT-1 cells compared with control siRNA-treated cells (Figure 3C). These results indicate that AnxA2 antibody inhibits the EGF-induced cell migration of MDA-MB-231 and JIMT-1 cells via EGFR. Previously, it has been shown that blocking AnxA2 function by AnxA2 antibody inhibits cell migration via tPA (Sharma et al, 2010). Additional experiments were performed to confirm whether inhibition of EGF-induced cell migration by AnxA2 antibody is dependent or independent of AnxA2-mediated plasmin generation via tPA. We further performed the wound-resealing assay in tPA-depleted JIMT-1 cells in the presence or absence of EGF and AnxA2 antibody. Our results clearly showed that depletion of tPA did not affect the EGF-induced cell migration of JIMT-1 cells compared with control siRNA-treated cells (Supplementary Figure S2A and B). After time-lapse imaging, we found that AnxA2 (D1/274.5) antibody preincubation resulted in 14.4% and 12.3% delay in EGF-induced wound closure after 24 h of wound formation in control and tPA siRNA-treated JIMT-1 (Supplementary Figure S2B) cells respectively, as compared with the control and with treatment with heat inactivated AnxA2 (D1/274.5) antibody. These experiments support a role of cell-surface AnxA2 in controlling EGFR function in triple-negative and Herceptin-resistant breast cancer cell migration in an EGF-dependent manner and this mechanism is independent of plasmin signalling.
AnxA2 antibody inhibits the EGF-induced cell migration of MDA-MB-231 and JIMT-1 cells. MDA-MB-231 (A) and JIMT-1 (B) cells were grown in 12-well plates. Wound-healing assay was performed. Bar graph showing the percentage wound closure after 24 h of wound formation. Data were collected from three independent experiments, and the mean±s.e. were calculated. (C) After 48 h of control and EGFR siRNA transfection in JIMT-1 cells, wound-healing assay was performed. Data were collected from three independent experiments, and the mean±s.e. were calculated. (*P<0.05; **P<0.01 vs control or heat inactivated AnxA2 antibody treatment group; #P insignificant).
AnxA2 antibody inhibits the EGF-induced EGFR homodimerisation and phosphorylation
Epidermal growth factor receptor is composed of an extracellular ligand-binding domain and a cytoplasmic C-terminal tyrosine kinase domain. Binding of ligands, such as EGF, to the extracellular domain of EGFR, induces the formation of homodimers, and leading to the autophosphorylation of tyrosine residues within the receptor’s cytoplasmic tail (Yarden and Sliwkowski, 2001; Lemmon and Schlessinger, 2010). First, we examined the effects of AnxA2 antibody pretreatment on EGF-induced homodimerisation of the EGFR by performing a crosslinking experiment in MDA-MB-231 or JIMT-1 cells. Compared with the respective controls, addition of EGF caused the dimerisation of EGFR in both cell types (Figure 4A). However, AnxA2 (D1/274.5) antibody pretreatment hindered the dimerisation of EGFR induced by EGF as compared with EGF alone or EGF with heat inactivated AnxA2 (D1/274.5) antibody pretreatment. To prove that inhibition of EGF-induced EGFR dimerisation was not an antibody-specific phenomenon limited to D1/274.5, we also used different monoclonal and polyclonal AnxA2 antibodies (Figure 4A). Our western blot analysis showed similar effects of inhibition of EGF-induced EGFR dimerisation upon pretreatment with AnxA2 antibodies in both cell types, as is the case with AnxA2 (D1/274.5) antibody pretreatment. The EGF-bound EGFR results in activation of tyrosine kinase activity and phosphorylation of multiple intracellular tyrosine residues (Yarden and Sliwkowski, 2001; Normanno et al, 2006; Lemmon and Schlessinger, 2010; Masuda et al, 2012). Therefore, we further examined the effect of pretreatment with AnxA2 antibodies on EGF-induced phosphorylation of individual EGFR tyrosine residues (Tyr-845, Tyr-1045, and Tyr-1068,) in MDA-MB-231 and JIMT-1 cells. When cells were stimulated with EGF, EGFR was phosphorylated at Tyr-845, Tyr-1045, and Tyr-1068 in both cell types (Figure 4B and C). However, pretreatment with different AnxA2 antibodies reduced the phosphorylation of EGFR at Tyr-845 in both cell types, while inhibition of EGFR phosphorylation at Tyr-1068 was only observed in JIMT-1 cells (Figure 4B and C). We did not find any change in phosphorylation of EGFR at Tyr-1045 in both cell types after pretreatment with AnxA2 antibodies (data not shown). However, we observed no change in total EGFR protein level after AnxA2 antibody pretreatment. Pretreatment with heat inactivated AnxA2 (D1/274.5) antibody did not affect the phosphorylation of EGFR as compared with EGF alone.
AnxA2 antibody inhibits the EGF-induced EGFR dimerisation and phosphorylation in MDA-MB-231 and JIMT-1 cells. (A) MDA-MB-231 and JIMT-1 cells were cultured overnight in medium without serum, and then stimulated with or without EGF (50 ng ml−1) for 20 min after 2 h of heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 antibody (2 μg ml−1) pretreatment. EGFR dimerisation was performed as described under ‘Experimental Procedures’ and analysed by western blotting. GAPDH was used as a loading control. BD, mouse anti-AnxA2 (clone 5) monoclonal antibody from BD Pharmingen (BD Biosciences, San Jose, CA); D1, mouse anti-AnxA2 (D1/274.5) monoclonal antibody; SC, rabbit anti-AnxA2 (H-50) polyclonal antibody from Santa Cruz Biotechnology (Santa Cruz, CA). (B) MDA-MB-231 and JIMT-1 cells were serum starved overnight, and then treated with or without EGF (50 ng ml−1) for 20 min after 2 h of heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 antibody (2 μg ml−1) pretreatment. The cell lysate was analysed for phosphorylated EGFR and EGFR by western blotting. (C) A bar graph showing densitometric analysis of bands used in (B). Each bar represents the mean±s.e. of three independent experiments.
AnxA2 antibody inhibits the EGF-induced EGFR internalisation
Binding of EGF to EGFR triggers rapid dimerisation and phosphorylation, followed by receptor internalisation and downregulation from the cell surface (Yarden and Sliwkowski, 2001; Normanno et al, 2006; Lemmon and Schlessinger, 2010; Masuda et al, 2012). To further examine whether AnxA2 antibody treatment also regulates the internalisation of the EGFR–ligand complex, we analysed EGF-induced EGFR internalisation using immunofluorescence microscopy. To check the EGFR internalisation, MDA-MB-231 and JIMT-1 cells were incubated with fluorescently labelled EGF (FITC-EGF) for 20 min after pretreatment with or without heat inactivated AnxA2 (D1/274.5) antibody or AnxA2 (D1/274.5) antibody. After treatment, cells were washed, fixed and immunostained with EEA1 marker to visualise the early endosomes, which is necessary for endosomal trafficking. In both control and heat inactivated AnxA2 antibody pretreated cells, EGF was internalised and showed a similar intracellular distribution, as evidenced by the colocalisation (yellow dots) of EGF with EEA1, while cells pretreated with AnxA2 (D1/274.5) antibody showed significantly less fluorescent EGF and colocalisation with EEA1 in both cell types, suggesting that AnxA2 antibody pretreatment inhibits the EGF-induced EGFR internalisation in MDA-MB-231 (Figure 5A and B) and JIMT-1 (Figure 5A and C) cells. To further confirm whether AnxA2 antibody treatment can regulate EGFR internalisation, we also performed flow-cytometry analysis to check the cell-surface expression of EGFR in MDA-MB-231 and JIMT-1 cells. As shown in Figure 5D and E, both control cell types were found to have relatively high amounts of EGFR expressed on the cell surface. Stimulation with EGF ligand in control and heat inactivated AnxA2 (D1/274.5) antibody pretreated cells showed a significant decrease in the levels of EGFR on the cell surface. In cells pretreated with AnxA2 (D1/274.5) antibody, although EGFR levels were reduced as compared with control, ligand stimulation did not significantly affect EGFR surface expression on MDA-MB-231 (Figure 5D) and JIMT-1 (Figure 5E) cells, indicating inhibition of internalisation.
AnxA2 antibody inhibits the EGF-induced EGFR internalisation in MDA-MB-231 and JIMT-1 cells. (A) The membrane function of AnxA2 was blocked by the treatment of AnxA2 (D1/274.5) antibody (2 μg ml−1) for 2 h after 12 h of serum starvation. MDA-MB-231 and JIMT-1 cells were treated with or without FITC labelled EGF (50 ng ml−1) for 20 min after AnxA2 antibody pretreatment. The cells were fixed, permeabilised, and incubated with rabbit anti-EEA1 antibody followed by anti-rabbit Alexa 595 secondary antibody. The slides were examined using LSM 510 Meta confocal system. The merged pictures of EGF (green), EEA1 (red) and nucleus (blue) are representative images of three independent experiments. Yellow dots represent the colocalisation of green and red colors, which indicates that EGF is colocalised with EEA1. Quantitative analysis of the percentage of colocalisation of EGF with EEA1 in MDA-MB-231 (B) and JIMT-1 (C) was calculated by analyzing a minimum of 10 cells from each treatment randomly taken from two independent experiments (***P<0.001 vs EGF or EGF+Heat inactivated AnxA2 antibody pretreatment). Inhibition of EGF-induced internalisation of EGFR at cell surface by AnxA2 antibody was measured by flow cytometry in MDA-MB-231 (D) and JIMT-1 (E) cells. The cells were incubated with or without EGF (50 ng ml−1) for 5 min after 2 h of heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) or AnxA2 (D1/274.5) antibody (2 μg ml−1) pretreatment. After fixation, cells were stained with anti-EGFR antibody without permeabilisation, followed by Alexa fluor 488-conjugated anti-rabbit IgG. After washing, cells were analysed by flow cytometry using the FITC signal detector (FL1) in Beckman Coulter FC500 flow cytometer. The data are plotted for cell numbers vs the intensity of fluorescence. Results are representative of two independent experiments.
AnxA2 antibody inhibits the EGF induced AKT pathway
Binding of EGF to the EGFR results in receptor dimerisation, autophosphorylation, and activation of various downstream signalling molecules including PI3K-PDK1-AKT pathway (Vivanco and Sawyers, 2002; Normanno et al, 2006), which has a role in its cell proliferation response. We next evaluated whether inhibition of EGF-induced cell proliferation by AnxA2 antibody is associated with reduced downstream kinase activity of AKT pathway. Functional activation of PI3K-PDK1-AKT pathway was evaluated by western blot analysis, using phospho-specific antibodies. As shown in Figure 6A and B, AnxA2 (D1/274.5) antibody pretreatment significantly inhibited the phosphorylation of p85 (Tyr-458) subunit of PI3K and AKT (Ser-473) in both cell types as compared with cells treated with EGF alone or EGF with heat inactivated AnxA2 (D1/274.5) antibody pretreatment. However, the downregulation of phospho-PDK1 (Ser-241) was observed only in JIMT-1 cells after AnxA2 antibody pretreatment. AnxA2 antibody treatment inhibits the phosphorylation of AKT with no effect on total AKT protein levels. To further determine whether EGF-induced phosphorylation of AKT was regulated via PI3K pathway, we examined the effect of the PI3K kinase inhibitor (LY294002) on phosphorylation of PDK1 and AKT in both cell types (Figure 6C). The EGF-induced phosphorylation of AKT (Ser-473) was inhibited by LY294002 in both cell types, which was similar to the results observed in AnxA2 antibody pretreated cells. However, consistent with our previous results, we did not observe the inhibition of phospho-PDK1 (Ser-241) in MDA-MB-231 cells, suggesting that AKT phosphorylation is directly regulated by PI3-Kinase activation.
AnxA2 antibody inhibits the EGF-induced AKT pathway in MDA-MB-231 and JIMT-1 cells. (A) MDA-MB-231 and JIMT-1 cells after 70% confluence were cultured overnight in serum free medium, and then incubated with EGF (50 ng ml−1) for 20 min after 2 h of heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) AnxA2 (D1/274.5) antibody (2 μg ml−1) pretreatment. Western blot analyses were carried out with indicated antibodies. (B) A bar graph showing densitometric analysis of bands used in (A). Each bar represents the mean±s.e. of three independent experiments. (C) After 12 h serum starvation, MDA-MB-231 and JIMT-1 cells were treated with or without EGF (50 ng ml−1) for 20 min after 2 h of PI3K inhibitor (10 μg ml−1) pretreatment. The cell lysate was analysed for p-PDK1 (Ser-241), p-AKT (Ser-473) by western blotting.
AnxA2 antibody inhibits the EGF-induced ERK pathway
Ligand binding to EGFR leads to the activation of mitogen-activated protein kinase (MAPK) cascades, which have important roles in the regulation of cell proliferation, survival, and differentiation (Normanno et al, 2006; Roberts and Der, 2007). The ERK pathway is one of the major signalling cassettes of the MAPK pathways. The ERK is a downstream component of an evolutionarily conserved signalling module that is activated by the Raf serine/threonine kinases. Raf activates the MAPK/ERK kinase (MEK) 1/2 dual-specificity protein kinases, which then activate ERK1/2. Therefore, we hypothesised a role for the Raf-MEK-ERK pathway in the inhibition of EGF-induced cell migration and proliferation in the presence of AnxA2 antibody. Western blot analysis (Figure 7A and B) showed that preincubation with AnxA2 (D1/274.5) antibody evidently reduced the expression levels of p-c-Raf (Ser-338) p-MEK1/2 (Ser-218/Ser-222), and p-ERK1/2 (Tyr-202/Tyr-204) but did not alter the expression levels of total ERK in MDA-MB-231 and JIMT-1 cells compared with EGF alone or EGF and preincubation with heat inactivated AnxA2 (D1/274.5) antibody. Although there is considerable experimental evidence that the Raf-MEK-ERK cascade is a critical mediator of Ras-induced oncogenesis (Roberts and Der, 2007), previous studies have clearly demonstrated that Ras also utilises additional effectors, including PI3K, to promote tumour proliferation (Yart et al, 2001; Sampaio et al, 2008). We therefore investigated whether activation of EGF-induced PI3K is associated with the activation of Raf-MEK-ERK signalling pathway. We examined the effect of the PI3K kinase inhibitor (LY294002) on phosphorylation of c-Raf-MEK-ERK pathway in the presence or absence of EGF in JIMT-1 cell lines. Results of these experiments showed that addition of PI3-kinase inhibitor (LY294002) nearly completely blocked the EGF-induced phosphorylation of c-Raf-MEK-ERK kinases (Figure 7C). Together, these results suggest that activation of Raf-MEK-ERK kinases is dependent on the activation of PI3K whose activity is regulated by EGFR in MDA-MB-231 and JIMT-1 breast cancer cell lines.
AnxA2 antibody inhibits the EGF-induced ERK pathway in MDA-MB-231 and JIMT-1 cells. (A) After 12 h serum starvation, cells were treated with or without EGF (50 ng ml−1) for 20 min after 2 h of heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1) AnxA2 (D1/274.5) antibody (2 μg ml−1) pretreatment. Western blot analyses were carried out with indicated antibodies. (B) A bar graph showing densitometric analysis of bands of protein used in (A). Each bar represents the mean±s.e. of three independent experiments. (C) After 12 h of serum starvation, JIMT-1 cells were treated with or without EGF (50 ng ml−1) for 20 min after 2 h of PI3K inhibitor (10 μg ml−1) pretreatment. The cell lysate was analysed for p-c-Raf (Ser-338), p-MEK (Ser-218/Ser-222), p-ERK (Thr-202/Tyr-204) by western blotting.
AnxA2 antibody treatment suppressed the proliferation and migration of MDA-MB-231 and JIMT-1 cells by inhibiting EGFR/AKT/ERK signalling pathways in full serum medium
In the above studies, we demonstrated that AnxA2 (D1/274.5) antibody preincubation inhibits EGF-induced EGFR dimerisation, tyrosine phosphorylation, internalisation, and its downstream signalling pathways PI3K-AKT, and Raf-MEK-ERK that leads to inhibition of cancer cell proliferation and migration. We further investigated whether AnxA2 (D1/274.5) antibody treatment inhibits the triple-negative and Herceptin-resistant breast cancer cell proliferation and migration in the basal growth medium in the presence of 10% serum, where different types of growth factors are present. Initially, to investigate the inhibition efficacy of AnxA2 (D1/274.5) antibody on cell proliferation in complete growth medium, MDA-MB-231 and JIMT-1 cells were cultured in the presence of AnxA2 (D1/274.5) antibody. After 4 days of treatment, inhibition of cell proliferation was measured by MTT assay. Compared with its control, cell proliferation was suppressed by 16% after AnxA2 (D1/274.5) antibody treatment in MDA-MB-231 cells (Figure 8A) and 22% growth inhibition was observed after AnxA2 (D1/274.5) antibody treatment in JIMT-1 cells (Figure 8B). The efficacy of AnxA2 (D1/274.5) antibody on inhibition of cell migration was further evaluated in MDA-MB-231 and JIMT-1 cells by scratch wound-healing assay in complete growth medium. Our results showed that migration was slower in both cell types (Figures 7D and 8C) after treatment with AnxA2 (D1/274.5) antibody as compared with control or treatment with heat inactivated AnxA2 (D1/274.5) antibody. After AnxA2 (D1/274.5) antibody treatment, ∼25% and 30% delay in wound closure was observed in MDA-MB-231 and JIMT-1 cells, respectively. To further validate that inhibition of cell proliferation and migration by AnxA2 (D1/274.5) antibody is associated with decreased phosphorylation of EGFR and its associated downstream signalling, we performed western blot analysis after treating the MDA-MB-231 and JIMT-1 cells with AnxA2 antibody in full serum medium at a concentration of 2 μg ml−1 per day for 4 days. Results presented in Figure 8E–G showed that AnxA2 (D1/274.5) antibody treatment inhibited the phosphorylation of EGFR at Try-845 and Tyr-1068 in both cell types. However, we did not observe any change in total EGFR expression in either cell type. Likewise, we found that treatment of AnxA2 (D1/274.5) antibody also reduced the phosphorylation of PI3K-AKT and Raf-MEK-ERK pathway in both cell types. Together, these results suggest that blocking the cell-surface AnxA2 function by AnxA2 antibody significantly inhibits the cell proliferation and migration via downregulation of EGFR phosphorylation and its associated downstream AKT and ERK signalling pathways. More importantly, these results suggest a role for AnxA2 in the regulation of EGFR-mediated cell proliferation and migration of triple-negative and Herceptin-resistant breast cancer.
AnxA2 antibody treatment suppressed the proliferation and migration of MDA-MB-231 and JIMT-1 cells by inhibiting EGFR/AKT/ERK signalling pathways in full serum medium. (A) MDA-MB-231 and (B) JIMT-1 cells (1 × 103) were plated in 96-well plates and treated with heat inactivated AnxA2 (D1/274.5) antibody (2 μg ml−1 per day) or AnxA2 (D1/274.5) antibody (2 μg ml−1 per day) for 4 days in full serum medium. After 4 days incubation, MTT assay was performed. Results presented are percent cell survival in treated groups with respect to control cells (mean±s.e.; n=8). For migration assay, MDA-MB-231 (C) and JIMT-1 (D) cells were grown in 12-well plates. After wound formation, cells were incubated with heat inactivated AnxA2 (D1/274.5) antibody (15 μg ml−1) or AnxA2 (D1/274.5) antibody (15 μg ml−1) in a full serum medium, and photographs were taken for different time intervals, as indicated. A bar graph showing the percentage wound closure in different time intervals after wound formation. Data were collected from two independent experiments, and the mean±s.e. were calculated (*P<0.05; **P<0.01 vs control or heat inactivated AnxA2 antibody treatment group). (E) Cells were treated with AnxA2 (D1/274.5) antibody (2 μg ml−1 per day) for 4 days in full serum medium. The cell lysate was analysed by western blotting with indicated antibodies. (F) MDA-MB-231 and (G) JIMT-1 cells, a bar graph showing densitometric analysis of bands of protein used in (E). Data were collected from two independent experiments, and the mean±s.e. were calculated.
Discussion
Although there is overexpression of AnxA2 in breast cancer and it has a significant association with metastatic disease (Sharma et al, 2006; Sharma and Sharma, 2007; Chuthapisith et al, 2009; Zhang et al, 2009; Shetty et al, 2012), limited information is available regarding its functional role in breast cancer, especially in the triple-negative and Herceptin-resistant breast cancer subtypes. The overexpression of AnxA2 in breast cancer has been shown to be capable of modulating key events in tumour progression; mainly those involving proliferation, adhesion, migration, invasion, angiogenesis, and drug resistance (Ling et al, 2004; Rescher and Gerke, 2004; Sharma and Sharma, 2007; Chuthapisith et al, 2009; Bharadwaj et al, 2013). In fact, AnxA2 is identified as a potential diagnostic and prognostic biomarker for prediction of tumour malignancy and for metastatic recurrence of breast cancer (Jeon et al, 2013). The results of the present study provide insight into the mechanisms involved in AnxA2 antibody mediated inhibition of cell proliferation and migration in triple-negative MDA-MB-231 and Herceptin-resistant JIMT-1 breast cancer cells. Previously, we have shown that AnxA2 is selectively overexpressed in TNBC and has an inverse correlation with HER2 expression (Shetty et al, 2012). Our results further confirmed that in addition to the increased expression at endogenous level, AnxA2 expression at the cell surface is elevated in triple-negative and Herceptin-resistant breast cancer cells, compared with other breast cancer subtypes, including non-tumourigenic epithelial cell lines. Enhanced AnxA2 expression has also been reported in hepatocellular carcinoma, pancreatic ductal adenocarcinoma, high-grade glioma, metastatic renal cell carcinoma, prostate cancer, advanced colorectal carcinoma, and acute promyelocytic leukemia (Vishwanatha et al, 1993; Banerjee et al, 2003; Zhang et al, 2012; Bharadwaj et al, 2013).
The AnxA2 interacts with receptor tyrosine kinases such as insulin receptor (IR), insulin-like growth factor receptor (IGFR), and EGFR to enhance tumour growth and metastases (Biener et al, 1996; Zhao et al, 2003; Shetty et al, 2012). Consistent with previous studies, our results confirm that a sub-population of AnxA2 present at the cell surface of triple-negative and Herceptin-resistant breast cancer cells interacts with EGFR. On the basis of the immunofluorescence studies, it appears that a sub-fraction of AnxA2 may go to the cell surface after EGF treatment. This indicates that EGF can affect the localisation of AnxA2 and subsequently increase its ability to bind with EGFR located on the external surface of the membrane. AnxA2 is a major substrate of pp60c-Src kinase and increases the phosphorylation of AnxA2 at Tyr-23, a site known for increasing cell-surface localisation of AnxA2 (Gerke and Moss, 2002). The EGF-induced cell-surface localisation of AnxA2 is obvious phenomenon as EGF stimulates the pp60c-Src kinase activity via EGFR (Jorissen et al, 2003). This cell surface localised binding may regulate an AnxA2-dependent EGFR signalling pathway. Consistent with this prediction, our results show that blocking cell-surface AnxA2 function by AnxA2 antibody significantly inhibits the EGF-induced proliferation and migration of triple-negative and Herceptin-resistant breast cancer cells. However, the existing literature also suggest that AnxA2 antibody inhibits cell migration via tPA by blocking plasmin generation (Sharma et al, 2010). In addition, plasmin is known to be a potent signalling molecule involved in migration, invasion, and angiogenesis process by activating ERK and AKT pathway (Zhang et al, 2007; Sharma et al, 2010). In contrast, our data indicate a clear role for AnxA2 antibody in inhibition of EGF-induced migration of triple-negative and Herceptin-resistant breast cancer cells in tPA-independent manner as depletion of tPA did not affect the EGF-induced migration of JIMT-1 cells.
Our results strongly suggest that AnxA2 is a limiting factor for EGF-induced dimerisation, tyrosine phosphorylation, and internalisation of EGFR in triple-negative and Herceptin-resistant breast cancer cells. We observed a decrease in EGF-induced EGFR phosphorylation at Tyr-845 and/or Tyr-1068 residue(s) in the presence of AnxA2 antibody, suggesting that AnxA2 regulates the overall function of EGFR, leading to the inhibition of cancer cell proliferation and migration. A plausible explanation for decreased EGF-induced receptor dimerisation and tyrosine phosphorylation could be that addition of AnxA2 (D1/274.5) antibody blocked AnxA2 function, resulting in the inhibition of EGF binding to EGFR and/or recruitment of other proteins which are critical for EGFR function. Since EGFR is a membrane protein and AnxA2 is known to be a lipid raft-associated scaffold protein (Rescher and Gerke, 2004; Valapala and Vishwanatha, 2011), we postulate that AnxA2 helps in bringing several proteins together to complex with EGFR on the lipid raft, hence activating the downstream signalling upon ligand stimulation. In contrast to our findings, it has been reported that knockdown of AnxA2 in MDA-MB-231 LM2 cells enhanced EGFR signalling via c-Jun N-terminal kinase and AKT downstream pathways and EGF-induced cell migration (de Graauw et al, 2014). Although the reasons for this dissimilarity are not yet clear, the inconsistency could be caused by differences in the genetic background of the cells. The reasons for this differential effect of AnxA2 in this cell line and MDA-MB-231 and JIMT-1 cell lines used in the present studies should be further investigated.
Addition of exogenous AnxA2 facilitates the growth of multiple myeloma and pancreatic cancer cells in vitro and in vivo by signalling through ERK and AKT pathway (Ortiz-Zapater et al, 2007; D’Souza et al, 2012). Both ERK and AKT activation have been previously shown to regulate breast cancer cell growth (Hoadley et al, 2007; Eccles, 2011). AnxA2-mediated tumourigenesis in breast cancer has been primarily suggested through this pathway by modulating the function of tyrosine kinase receptor (Grewal and Enrich, 2009). However, our present study demonstrates that addition of AnxA2 antibody significantly inhibits the EGFR-dependent downstream PI3K-AKT and Raf-MEK-ERK pathway under both EGF-induced and basal growth condition in triple-negative and Herceptin-resistant breast cancer cells. It has been shown that ligand activation of EGFR stimulates PI3K activation, which further activates the signalling of PDK1-AKT and Raf-MEK-ERK pathway that regulates cell survival, proliferation, migration, and cellular metabolism in multiple tumour types (King et al, 1997; Yart et al, 2001; Vivanco and Sawyers, 2002; Normanno et al, 2006; Sampaio et al, 2008). Our results demonstrate that addition of AnxA2 antibody significantly inhibits the phosphorylation of regulatory subunit of PI3K (p85) on Tyr-458 residue, a site that has previously been reported to track the activation of PI3K (Kim et al, 2006; Warfel et al, 2011), under both EGF-induced and basal growth conditions. Furthermore, treatment with AnxA2 antibody suppressed the phosphorylation of AKT at Ser-473 in MDA-MB-231 cells and PDK1 and AKT at Ser-241 and Ser-473, respectively, in JIMT-1 cells under both EGF-induced and basal growth conditions. Consistent with this observation, preincubation with PI3K inhibitor did not suppress the EGF-induced PDK1 phosphorylation at Ser-241 residue in MDA-MB-231 cells. These observations suggest that AKT phosphorylation is directly regulated by PI3K activation at the inner surface of cell membrane in TNBC cells (Vivanco and Sawyers, 2002). Moreover, our results with AnxA2 antibody treatment or with a PI3K inhibitor show that activation of c-Raf-MEK-ERK pathway is significantly inhibited under EGF-induced or basal growth conditions in both cell types. It was previously shown that EGF-induced stimulation of PI3K is involved in the activation of Raf-MEK-ERK via Ras oncogene (King et al, 1997; Yart et al, 2001; Sampaio et al, 2008). Taken together, these findings strongly suggest that EGF-induced PI3K activation is central to both pathways and that its kinase activity could be attenuated by AnxA2 antibody by modulating EGFR functions in triple-negative and Herceptin-resistant breast cancer.
In conclusion, our results strongly suggest that addition of AnxA2 antibody causes the inhibition of proliferation and migration in triple-negative and Herceptin-resistant breast cancer cells via blocking EGFR functions as presented in the model shown in Figure 9. Blocking AnxA2 functions at the cell surface by AnxA2 antibody suppressed the tyrosine phosphorylation and internalisation of EGFR by blocking its homodimerisation, leading to the downregulation of ligand mediated EGFR downstream AKT and ERK signalling pathways. Our findings suggest that high expression of cell-surface AnxA2 in triple-negative and Herceptin-resistant breast cancer has an important compensatory and regulatory role via the EGFR-mediated oncogenic processes by keeping EGFR signalling events in an activated state. Therefore, AnxA2 could potentially be used as an important therapeutic target in triple-negative and Herceptin-resistant breast cancers.
Proposed model for inhibition of EGF-induced EGFR function by AnxA2 antibody in triple-negative and Herceptin-resistant breast cancer. AnxA2 and EGFR recruitment at cell surface is important for EGFR function that regulates cell proliferation and migration. Blocking AnxA2 function at the cell surface inhibits the EGF-induced EGFR homodimerisation, tyrosine phosphorylation, internalisation and EGFR-mediated downstream AKT and ERK signalling pathways resulting in inhibition of cell proliferation and migration in triple-negative and Herceptin-resistant breast cancer cells.
Change history
09 December 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Banerjee AG, Liu J, Yuan Y, Gopalakrishnan VK, Johansson SL, Dinda AK, Gupta NP, Trevino L, Vishwanatha JK (2003) Expression of biomarkers modulating prostate cancer angiogenesis: differential expression of annexin II in prostate carcinomas from India and USA. Mol Cancer 2: 34.
Bharadwaj A, Bydoun M, Holloway R, Waisman D (2013) Annexin A2 heterotetramer: structure and function. Int J Mol Sci 14: 6259–6305.
Biener Y, Feinstein R, Mayak M, Kaburagi Y, Kadowaki T, Zick Y (1996) Annexin II is a novel player in insulin signal transduction. Possible association between annexin II phosphorylation and insulin receptor internalization. J Biol Chem 271: 29489–29496.
Chuthapisith S, Bean BE, Cowley G, Eremin JM, Samphao S, Layfield R, Kerr ID, Wiseman J, El-Sheemy M, Sreenivasan T, Eremin O (2009) Annexins in human breast cancer: possible predictors of pathological response to neoadjuvant chemotherapy. Eur J Cancer 45: 1274–1281.
Chuthapisith S, Layfield R, Kerr ID, Hughes C, Eremin O (2007) Proteomic profiling of MCF-7 breast cancer cells with chemoresistance to different types of anti-cancer drugs. Int J Oncol 30: 1545–1551.
de Graauw M, Cao L, Winkel L, van Miltenburg MH, le Dévédec SE, Klop M, Yan K, Pont C, Rogkoti VM, Tijsma A, Chaudhuri A, Lalai R, Price L, Verbeek F, van de Water B (2014) Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene 33: 2610–2619.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13: 4429–4434.
D’Souza S, Kurihara N, Shiozawa Y, Joseph J, Taichman R, Galson DL, Roodman GD (2012) Annexin II interactions with the annexin II receptor enhance multiple myeloma cell adhesion and growth in the bone marrow microenvironment. Blood 119: 1888–1896.
Eccles SA (2011) The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol 55: 685–696.
Flood EC, Hajjar KA (2011) The annexin A2 system and vascular homeostasis. Vascul Pharmacol 54: 59–67.
Grewal T, Enrich C (2009) Annexins-modulators of EGF receptor signalling and trafficking. Cell Signal 21: 847–858.
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82: 331–371.
Hoadley KA, Weigman VJ, Fan C, Sawyer LR, He X, Troester MA, Sartor CI, Rieger-House T, Bernard PS, Carey LA, Perou CM (2007) EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics 8: 258.
Hurvitz SA, Finn RS (2009) What's positive about 'triple-negative' breast cancer? Future Oncol 5: 1015–1025.
Jeon YR, Kim SY, Lee EJ, Kim YN, Noh DY, Park SY, Moon A (2013) Identification of annexin II as a novel secretory biomarker for breast cancer. Proteomics 13: 3145–3156.
Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW (2003) Epidermal growth factor receptor: mechanisms of activation and signaling. Exp Cell Res 284: 31–53.
Kesavan K, Ratliff J, Johnson EW, Dahlberg W, Asara JM, Misra P, Frangioni JV, Jacoby DB (2010) Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem 285: 4366–4374.
Kim JH, Xu C, Keum YS, Reddy B, Conney A, Kong AN (2006) Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethylisothiocyanate and curcumin. Carcinogenesis 27: 475–482.
King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS (1997) Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol 17: 4406–4418.
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134.
Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA (2004) Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest 113: 38–48.
Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT (2012) Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 136: 331–345.
Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366: 2–16.
Ortiz-Zapater E, Peiró S, Roda O, Corominas JM, Aguilar S, Ampurdanés C, Real FX, Navarro P (2007) Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am J Pathol 170: 1573–1584.
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62: 233–247.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406: 747–752.
Pohlmann PR, Mayer IA, Mernaugh R (2009) Resistance to trastuzumab in breast cancer. Clin Cancer Res 15: 7479–7491.
Rescher U, Gerke V (2004) Annexins–unique membrane binding proteins with diverse functions. J Cell Sci 117: 2631–2639.
Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26: 3291–3310.
Sampaio C, Dance M, Montagner A, Edouard T, Malet N, Perret B, Yart A, Salles JP, Raynal P (2008) Signal strength dictates phosphoinositide 3-kinase contribution to Ras/extracellular signal-regulated kinase 1 and 2 activation via differential Gab1/Shp2 recruitment: consequences for resistance to epidermal growth factor receptor inhibition. Mol Cell Biol 28: 587–600.
Sharma MC, Sharma M (2007) The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des 13: 3568–3575.
Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC (2006) Angiogenesis-associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol 81: 146–156.
Sharma M, Blackman MR, Sharma MC (2012) Antibody-directed neutralization of annexin II (ANX II) inhibits neoangiogenesis and human breast tumor growth in a xenograft model. Exp Mol Pathol 92: 175–184.
Sharma M, Ownbey RT, Sharma MC (2010) Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol 88: 278–286.
Shetty PK, Thamake SI, Biswas S, Johansson SL, Vishwanatha JK (2012) Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS One 7: e44299.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98: 10869–10874.
Stevens KN, Vachon CM, Couch FJ (2013) Genetic susceptibility to triple-negative breast cancer. Cancer Res 73: 2025–2030.
Tuszynski GP, Sharma MR, Rothman VL, Sharma MC (2002) Angiostatin binds to tyrosine kinase substrate annexin II through the lysine-binding domain in endothelial cells. Microvasc Res 64: 448–462.
Valapala M, Vishwanatha JK (2011) Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem 286: 30911–30925.
Valapala M, Thamake SI, Vishwanatha JK (2011a) A competitive hexapeptide inhibitor of annexin A2 prevents hypoxia-induced angiogenic events. J Cell Sci 124: 1453–1464.
Vishwanatha JK, Chiang Y, Kumble KD, Hollingsworth MA, Pour PM (1993) Enhanced expression of annexin II in human pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis 14: 2575–2579.
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2: 489–501.
Warfel NA, Niederst M, Newton AC (2011) Disruption of the interface between the pleckstrin homology (PH) and kinase domains of Akt protein is sufficient for hydrophobic motif site phosphorylation in the absence of mTORC2. J Biol Chem 286: 39122–39129.
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137.
Yart A, Laffargue M, Mayeux P, Chretien S, Peres C, Tonks N, Roche S, Payrastre B, Chap H, Raynal P (2001) A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J Biol Chem 276: 8856–8864.
Yehiely F, Moyano JV, Evans JR, Nielsen TO, Cryns VL (2006) Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med 12: 537–544.
Zhang F, Zhang L, Zhang B, Wei X, Yang Y, Qi RZ, Ying G, Zhang N, Niu R (2009) Anxa2 plays a critical role in enhanced invasiveness of the multidrug resistant human breast cancer cells. J Proteome Res 8: 5041–5047.
Zhang Y, Zhou ZH, Bugge TH, Wahl LM (2007) Urokinase-type plasminogen activator stimulation of monocyte matrix metalloproteinase-1 production is mediated by plasmin-dependent signaling through annexin A2 and inhibited by inactive plasmin. J Immunol 179: 3297–3304.
Zhang X, Liu S, Guo C, Zong J, Sun MZ (2012) The association of annexin A2 and cancers. Clin Transl Oncol 14: 634–640.
Zhao WQ, Chen GH, Chen H, Pascale A, Ravindranath L, Quon MJ, Alkon DL (2003) Secretion of Annexin II via activation of insulin receptor and insulin-like growth factor receptor. J Biol Chem 278: 4205–4215.
Acknowledgements
This work was supported by National Institutes of Health Grant 1P20 MD006882.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Chaudhary, P., Thamake, S., Shetty, P. et al. Inhibition of triple-negative and Herceptin-resistant breast cancer cell proliferation and migration by Annexin A2 antibodies. Br J Cancer 111, 2328–2341 (2014). https://doi.org/10.1038/bjc.2014.542
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.542