Abstract
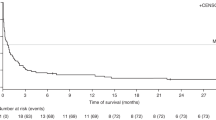
We analyzed the incidence, clinicopathological features, risk factors and prognosis of patients with EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD) in 288 adults undergoing umbilical cord blood transplantation (UCBT) at a single institution. Twelve patients developed proven EBV-PTLD at a median time of 73 days (range, 36–812). Three-year cumulative incidence (CI) of EBV-PTLD was 4.3% (95% CI: 1.9–6.7). All patients presented with extranodal involvement. Most frequently affected sites were the liver, spleen, central nervous system (CNS), Waldeyer’s ring and BM in 7, 6, 4, 3 and 3 patients, respectively. One patient had polymorphic and 11 had monomorphic EBV-PTLD (7 diffuse large B-cell lymphomas not otherwise specified, 4 plasmablastic lymphomas). We confirmed donor origin and EBV infection in all histological samples. EBV-PTLD was the cause of death in 11 patients at a median time of 23 days (range, 1–84). The 3-year CI of EBV-PTLD was 12.9% (95% CI: 3.2–22.5) and 2.6% (95% CI: 0.5–4.7) for patients receiving reduced-intensity conditioning (RIC) and myeloablative conditioning, respectively (P<0.0001). In conclusion, adults with EBV-PTLD after UCBT showed frequent visceral and CNS involvement. The prognosis was poor despite routine viral monitoring and early intervention. An increased risk of EBV-PTLD was noted among recipients of RIC regimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Curtis RE, Travis LB, Rowlings PA, Socié G, Kingma DW, Banks PM et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 1999; 94: 2208–2216.
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik J-A et al. Marked increased risk of Epstein–Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006; 108: 2874–2880.
Dumas P-Y, Ruggeri A, Robin M, Crotta A, Abraham J, Forcade E et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: a Eurocord and Société Francaise de Greffede Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant 2013; 48: 253–256.
Sanz J, Boluda JCH, Martín C, González M, Ferrá C, Serrano D et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant 2012; 47: 1287–1293.
Sanz J, Sanz MA, Saavedra S, Lorenzo I, Montesinos P, Senent L et al. Cord blood transplantation from unrelated donors in adults with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant 2010; 16: 86–94.
Montesinos P, Sanz J, Cantero S, Lorenzo I, Martín G, Saavedra S et al. Incidence, risk factors, and outcome of cytomegalovirus infection and disease in patients receiving prophylaxis with oral valganciclovir or intravenous ganciclovir after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2009; 15: 730–740.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757–770.
Swerdlow SH . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4th edn International Agency for Research on Cancer, World Health Organization: Lyon, France, 2008.
Moscardó F, Sanz J, Senent L, Cantero S, de la Rubia J, Montesinos P et al. Impact of hematopoietic chimerism at day +14 on engraftment after unrelated donor umbilical cord blood transplantation for hematologic malignancies. Haematologica 2009; 94: 827–832.
Gooley T, Leisenring W, Crowley J, Storer B . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 665–706.
Fine J, Gray R . A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2012. ISBN 900051-07-0. http://www.R-project.org.
Cohen J, Gandhi M, Naik P, Cubitt D, Rao K, Thaker U et al. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br J Haematol 2005; 129: 229–239.
Micallef IN, Chhanabhai M, Gascoyne RD, Shepherd JD, Fung HC, Nantel SH et al. Lymphoproliferative disorders following allogeneic bone marrow transplantation: the Vancouver experience. Bone Marrow Transplant 1998; 22: 981–987.
Penn I, Porat G . Central nervous system lymphomas in organ allograft recipients. Transplantation 1995; 59: 240–244.
Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc 2005; 37: 954–955.
Sirvent-Von Bueltzingsloewen A, Sirvent N, Morand P, Cassuto JP . Fatal central nervous system lesions emerging during anti-CD20 monoclonal antibody therapy (Rituximab) for a post transplantation Epstein–Barr virus-linked lymphoma. Med Pediatr Oncol 2003; 40: 408–409.
Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 4992–5001.
Liu JJ, Zhang L, Ayala E, Field T, Ochoa-Bayona JL, Perez L et al. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res 2011; 35: 1571–1577.
Chen DB, Song QJ, Chen YX, Chen YH, Shen DH . Clinicopathologic spectrum and EBV status of post-transplant lymphoproliferative disorders after allogeneic hematopoietic stem cell transplantation. Int J Hematol 2013; 97: 117–124.
Ballen KK, Cutler C, Yeap BY, McAfee SL, Dey BR, Attar EC et al. Donor-derived second hematologic malignancies after cord blood transplantation. Biol Blood Marrow Transplant 2010; 16: 1025–1031.
Heslop HE . How I treat EBV lymphoproliferation. Blood 2009; 114: 4002–4008.
Jagadeesh D, Woda BA, Draper J, Evens AM . Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol 2012; 13: 122–136.
van Burik J-A, Brunstein CG . Infectious complications following unrelated cord blood transplantation. Vox Sang 2007; 92: 289–296.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115: 925–935.
Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010; 116: 5045–5049.
Johansson JE, Remberger M, Lazarevic VLj, Hallböök H, Wahlin A, Kimby E et al. Allogeneic haematopoietic stem-cell transplantation with reduced intensity conditioning for advanced stage Hodgkin’s lymphoma in Sweden: high incidence of post transplantlymphoproliferative disorder. Bone Marrow Transplant 2011; 46: 870–875.
Thorley-Lawson DA, Gross A . Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N Engl J Med 2004; 350: 1328–1337.
Gandhi MK, Lambley E, Burrows J, Dua U, Elliott S, Shaw PJ et al. Plasma Epstein–Barr virus (EBV) DNA is a biomarker for EBV-positive Hodgkin’s lymphoma. Clin Cancer Res 2006; 12: 460–464.
Acknowledgements
We thank David Pellicer and Shirley Weiss for data collection and management. JS and GFS conceived the study; JS and MA interpreted the data; JS, MA and MAS wrote the paper; JS performed the statistical analyses; LS, AS and EM performed histopathological, phenotypic and molecular analysis; JLL-H performed EBV PCR tests; LS, IJ, PM, AS, IL, GM, FM, EM, MS, CC, BB, CS, JLL-H and GFS reviewed the manuscript and contributed to the final draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sanz, J., Arango, M., Senent, L. et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant 49, 397–402 (2014). https://doi.org/10.1038/bmt.2013.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.190
Keywords
This article is cited by
-
Rituximab for posttransplant lymphoproliferative disorder – therapeutic, preemptive, or prophylactic?
Bone Marrow Transplantation (2024)
-
Pre-emptive rituximab treatment for Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation is a worthwhile strategy in high-risk recipients: a comparative study for immune recovery and clinical outcomes
Bone Marrow Transplantation (2020)
-
Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes
Bone Marrow Transplantation (2020)
-
Post Transplant Lymphoproliferative Disorder
Indian Journal of Hematology and Blood Transfusion (2020)
-
A retrospective analysis on anti-CD20 antibody–treated Epstein–Barr virus–related posttransplantation lymphoproliferative disorder following ATG-based haploidentical T-replete hematopoietic stem cell transplantation
Annals of Hematology (2020)