Abstract
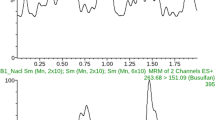
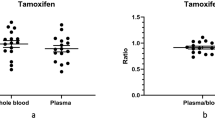
Individual therapeutic monitoring of busulfan (BU) minimizes its toxicity and improves the therapeutic outcomes during hematopoietic stem cell transplantation (HSCT). For individual dose adjustment, several blood collections are performed that are uncomfortable for patients. The aim of this pilot study was to validate a laboratory method for quantification of BU in saliva and to present the results obtained using this protocol in HSCT patients. We performed analyses of selectivity, precision and accuracy of saliva with standard concentrations of BU using ultra-high-performance liquid chromatography with diode array detection. We also determined salivary and plasmatic concentrations of BU in six HSCT patients. Saliva exhibited excellent selectivity, precision and accuracy for quantification of BU. In the patient samples, significant correlations were noted between plasmatic and salivary concentrations of BU (r=0.97, P<0.001 in the test dose; r=0.93, P<0.001 in the adjusted dose). Passing & Bablok regression revealed good agreement between the two methods (R2=0.956 for test dose; R2=0.927 for adjusted dose). In conclusion, the saliva is safe for laboratory BU measurement. The good agreement with plasma encourages further clinical studies using saliva for BU therapeutic monitoring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8: 468–476.
Sobecks RM, Rybicki L, Yurch M, Kalaycio M, Dean R, Andresen S et al. Intravenous compared with oral busulfan as preparation for allogeneic hematopoietic progenitor cell transplantation for AML and MDS. Bone Marrow Transplant 2012; 47: 633–638.
Russell JA, Kangarloo SB . Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des 2008; 14: 1936–1949.
Rauh M, Stachel D, Kuhlen M, Gröschl M, Holter W, Rascher W . Quantification of busulfan insaliva and plasma in haematopoietic stem cell transplantation in children: validation of liquid chromatography tandem mass spectrometry method. Clin Pharmacokinet 2006; 45: 305–316.
Ansari M, Uppugunduri CR, Déglon J, Théorêt Y, Versace F, Gumy-Pause F et al. A simplified method for busulfan monitoring using dried blood spot in combination with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2012; 26: 1437–1446.
Heggie JR, Wu M, Burns RB, Ng CS, Fung HC, Knight G et al. Validation of a high-performance liquid chromatographic assay method for pharmacokinetic evaluation of busulfan. J Chromatogr B Biomed Sci Appl 1997; 692: 437–444.
Hara S, Tsuchie M, Tsujioka R, Kimura M, Fujii M, Kuroda T et al. High-performance liquid chromatographic quantification of busulfan in human serum after fluorescence derivatization by 2-naphthalenethiol. Anal Sci 2000; 16: 287–291.
Burns RB, Heggie JR, Embree L . A gas-chromatographic assay method for busulfan with sensitivity for test dose therapeutic monitoring. J Pharm Biomed Anal 1995; 13: 1073–1078.
Lai WK, Pang CP, Law LK, Wong R, Li CK, Yuen PM . Routine analysis of plasma busulfan by gas chromatography-mass fragmentography. Clin Chem 1998; 44: 2506–2510.
Abdel-Rehim M, Hassan Z, Blomberg L, Hassan M . On-line derivatization utilizing solid-phase microextraction (SPME) for determination of busulphan in plasma using gas chromatography-mass spectrometry (GC-MS). Ther Drug Monit 2003; 25: 400–406.
Raju KS, Taneja I, Singh SP, Wahajuddin . Utility of noninvasive biomatrices in pharmacokinetic studies. Biomed Chromatogr 2013; 27: 1354–1366.
Gibaldi M, Levy G . Pharmacokinetics in clinical practice. 2. Applications. JAMA 1976; 235: 1987–1992.
Zhang Y, Huo M, Zhou J, Xie S . PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 2010; 99: 306–314.
Mauramo M, Rohde L, Ramseier AM, Rovó A, Waltimo T . Determinants of stimulated salivary flow among haematopoietic stem cell transplantation recipients. Clin Oral Investig 2016; 21: 121–126.
Ehrsson H, Hassan M . Binding of busulfan to plasma proteins and blood cells. J Pharm Pharmacol 1984; 36: 694–696.
Nath CE, Shaw PJ . Busulphan in blood and marrow transplantation: dose, route, frequency and role of therapeutic drug monitoring. Curr Clin Pharmacol 2007; 2: 75–91.
Aps JK, Martens LC . The physiology of saliva and transfer of drugs into saliva. Forensic Sci Int 2005; 150: 119–131.
Hassan M, Ehrsson H, Smedmyr B, Tötterman T, Wallin I, Oberg G et al. Cerebrospinal fluid and plasma concentrations of busulfan during high-dose therapy. Bone Marrow Transplant 1989; 4: 113–114.
Carpenter GH . The secretion, components, and properties of saliva. Annu Rev Food Sci Technol 2013; 4: 267–276.
Meurman JH, Laine P, Keinànen S, Pyrhönen S, Teerenhovi L, Lindqvist C . Five-year follow-up of saliva in patients treated for lymphomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 83: 447–452.
Karolewska E, Konopka T, Pupek M, Chybicka A, Mendak M . Antibacterial potential of saliva in children with leukemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105: 739–744.
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant 2008; 14: 220–228.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Bezinelli, L., Eduardo, F., de Carvalho, D. et al. Therapeutic salivary monitoring of IV busulfan in patients undergoing hematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant 52, 1384–1389 (2017). https://doi.org/10.1038/bmt.2017.142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.142
This article is cited by
-
Assessment of bone metabolism biomarkers in serum and saliva of cirrhotic patients
Clinical Oral Investigations (2022)
-
Nitrogenous compounds in the saliva and blood of cirrhotic patients: a cross-sectional study
Clinical Oral Investigations (2022)
-
Retrospective study of the digestive tract mucositis derived from myeloablative and non-myeloablative/reduced-intensity conditionings with busulfan in hematopoietic cell transplantation patient
Supportive Care in Cancer (2019)