Abstract
Childhood neuroblastic tumors are characterized by heterogeneous clinical courses, ranging from benign ganglioneuroma (GN) to highly lethal neuroblastoma (NB). Although a refined prognostic evaluation and risk stratification of each tumor patient is becoming increasingly essential to personalize treatment options, currently only few biomolecular markers (essentially MYCN amplification, chromosome 11q status and DNA ploidy) are validated for this purpose in neuroblastic tumors. Here we report that Galectin-3 (Gal-3), a β-galactoside-binding lectin involved in multiple biological functions that has already acquired diagnostic relevance in specific clinical settings, is variably expressed in most differentiated and less aggressive neuroblastic tumors, such as GN and ganglioneuroblastoma, as well as in a subset of NB cases. Gal-3 expression is associated with the INPC histopathological categorization (P<0.001) and Shimada favorable phenotype (P=0.001), but not with other prognostically relevant features. Importantly, Gal-3 expression was associated with a better 5-year overall survival (P=0.003), and with improved cumulative survival in patient subsets at worse prognosis, such as older age at diagnosis, advanced stages or NB histopathological classification. In vitro, Gal-3 expression and nuclear accumulation accompanied retinoic acid-induced cell differentiation in NB cell lines. Forced Gal-3 overexpression increased phenotypic differentiation and substrate adherence, while inhibiting proliferation. Altogether, these findings suggest that Gal-3 is a biologically relevant player for neuroblastic tumors, whose determination by conventional immunohistochemistry might be used for outcome assessment and patient’s risk stratification in the clinical setting.
Similar content being viewed by others
Main
Neuroblastic tumors (NTs) are embryonal neoplasms derived from neural crest precursors developing in the sympathetic nervous system. They are very complex and clinically heterogeneous diseases, ranging from spontaneously regressing tumors to highly aggressive and inexorable neoplasms.
At the molecular level, MYCN represents the most relevant player, biologically and prognostically. Indeed, its overexpression in mice is sufficient to induce neuroblastoma (NB) development.1 Its genomic amplification, occurring in about 20–25% of NB cases, is the most relevant marker of poor outcome in humans.2, 3 Somatic mutations in specific oncogenes or tumor suppressor genes, besides MYCN, appear to occur rather rarely in NB as compared with other tumors,4 limiting the identification of novel potential biomarkers for risk class stratification and the feasibility of genetic-driven targeted therapies. On the basis of the International Neuroblastoma Risk Group (INRG) consensus, seven independent prognostic indices (stage, age, histologic category, grade of tumor differentiation, MYCN amplification status, chromosome 11q status and DNA ploidy) are commonly used for the stratification of patients affected with NTs into defined risk groups.5 Although additional biological variables were eventually shown to possess prognostic value,6 their strong linkage to established prognostic factors (i.e., NTRK1 and NTRK2 expression),7 or their complex and expensive determination methods (i.e., gene profiling or microRNA signatures),8, 9, 10 has prevented their routine application for outcome assessment and risk class stratification in the clinical setting.
Currently, histopathological classification of NTs remains of paramount importance for risk definition. According to the International Neuroblastoma Pathology Committee (INPC) and based on the differentiation status of tumor cells and abundance of Schwannian stroma, NTs might be divided into four morphological categories with subtypes:11, 12 (1) NB (Schwannian stroma-poor) undifferentiated, poorly differentiated and differentiating; (2) ganglioneuroblastoma (GNB) intermixed (Schwannian stroma-rich); (3) ganglioneuroma (GN, Schwannian stroma-dominant) maturing and mature; (4) GNB nodular (composite Schwannian stroma-rich/stroma-dominant/Schwannian stroma-poor). By adding to the above the mitosis-karyorrhexis index in conjunction with age, the extensively used Shimada System efficaciously dichotomizes NTs into two risk categories: favorable and unfavorable.11, 12
Inconsistent results have either supported or denied the origin of Schwannian and neuroblastic cells from a common tumor-initiating cell.13, 14, 15, 16 Furthermore, a possible ‘reactive nature’ of Schwannian cells populating NTs has been proposed.17 Thus, although Schwannian stroma represents a biologically relevant feature, its nature is still controversial.
Galectin-3 (Gal-3) is a β-galactoside-binding lectin with multiple functions in relevant biological processes including premRNA processing, cell cycle progression, cell adhesion, angiogenesis, apoptosis, cell migration and inflammation.18 Its segregation in particular cellular compartments (i.e., nucleus, cytoplasm or mitochondria) or its secretion in the extracellular matrix largely dictates Gal-3 functions.19, 20, 21 In human cancer, either increased or decreased Gal-3 levels have been detected in more advanced tumor stages and are associated with reduced survival, depending on tumor histology.22, 23, 24, 25, 26, 27 With regard to the nervous system, both glial cells and few subsets of sensory neurons express Gal-3. Furthermore, Gal-3 is highly expressed in Schwannian cells of injured nervous fibers28, 29 and secreted Gal-3 impairs Schwannian cell proliferation and promotes neural cell adhesion and neurite growth.30, 31 Little is known on Gal-3 expression and function in the tumors of the nervous system, with little information being available for gliomas.32 We recently reported that Gal-3 is regulated by MYCN and is involved in resistance to apoptosis, in human NB cells.33, 34 Interestingly, we also reported marked differences in Gal-3 expression levels and subcellular localization between MYCN-amplified neuroblastic (N-type) cell lines and MYCN single-copy and substrate-adherent (S-type) cell lines.34
In the present study, we investigated on Gal-3 expression in a series of primary human NTs ex vivo and assessed the biological role played by Gal-3 in regulating NB cell proliferation, differentiation and adherence, in experimental NB tumor models, in vitro.
Results
Different patterns of Gal-3 expression in NTs
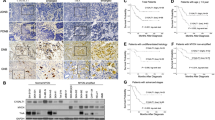
Prompted by our observations on Gal-3 expression in NB cell lines,34 we evaluated its expression in a multi-institutional series of 128 primary NTs. Histopathological examination classified NTs into NBs (104), GNBs (19) and GN (5). Additional clinical and pathological information is summarized in Supplementary Table 1. Immunohistochemical analysis for Gal-3 expression revealed readily detectable positivity in at least 10% of the cells in 22.7% (29/128) of the tumor samples (Supplementary Table S1). Gal-3 expression consistently appeared in two different patterns. In 100% (5/5) of the GN cases, Gal-3 expression was detected in the nuclei and cytoplasms of the majority of Schwannian stromal cells (Figure 1a and Table 1), identified according to their morphology and S-100 staining (Supplementary Figures S1A and B). We found the same pattern of expression in 91% (11/12) of the Gal-3-positive GNB cases, and in 50% (6/12) of the Gal-3-positive NB cases (Table 1, Figure 1b and Supplementary Table S1). In sharp contrast, six NB cases and one GNB case exhibited a Gal-3 staining restricted to the cytoplasm of immature neuroblasts and eventually to the neuropils, with undetectable nuclear staining (Supplementary Table S1 and Figures 1c and d). Gal-3 expression was not an obligate feature of Schwannian stromal cells, as we observed S-100-positive and Gal-3-negative Schwannian stromal cells in several NB cases (Supplementary Figures S1C and D), which indicates that qualitatively and/or biologically different Schwannian stromal cells exist in NTs. Finally, mature ganglion cells were invariably Gal-3-negative whenever they appeared in the sections (Figures 1a and b).
Gal-3 expression in primary human NTs. Immunohistochemical analysis shows that mAb to Gal-3 strongly stained nuclei and cytoplasms of the majority of the Schwannian stromal cells in all GN cases, in most GNB cases and in a few NB cases, as exemplified by GN case number 55 (a) or NBd case number10. (b) In contrast, in some NBs, Gal-3 expression was restricted to the cytoplasm of immature neuroblasts (with nuclear exclusion) and in the neuropils, as in NB cases number 29 and number 25. (c, d) Gal-3 antibody never stained ganglion cells. (Direct immunoperoxidase with HRP-conjugated mAb to Galectin-3; DAB as chromogenic substrate; counterstained with hematoxilin)
Gal-3 expression associates with positive prognostic factors in NTs
Consistent with previous observations,34 there was a trend for Gal-3-positive staining to be inversely correlated with MYCN amplification status (MNA), with only 9.7% of the MNA cases being positive for Gal-3 compared with 23.6% of the MYCN single-copy cases (Table 1). Interestingly, Gal-3 expression significantly associated with the INPC histopathological categorization of the tumors, with 100% of the GN and 63.2% of the GNB cases being positive for Gal-3 immunostaining, as compared with only 11.5% of the NB cases (Table 1). Furthermore, the rate of Gal-3 positivity significantly declined in poorly differentiated/undifferentiated NBs compared with differentiating NBs (Table 1). Gal-3 expression significantly correlated also with Shimada favorable phenotype (32.9 versus 9.7%; P=0.001) (Table 1), suggesting possible implications for its use for outcome assessment of NTs in the clinical setting. Gal-3 expression did not associate with other prognostically relevant features such as age at diagnosis and stage.
Gal-3 expression is a marker of favorable prognosis in NTs
As expected, age at diagnosis, stage, MNA status and Shimada category were all significant prognostic factors in this series (Table 2). Interestingly, Gal-3 expression also showed a strong and significant association with a better 5-year overall survival (OS, Table 2). Furthemore, Gal-3 expression predicted a significantly improved cumulative survival in patient subsets with a worse prognosis, such as older age at diagnosis (⩾15 months), advanced (3–4) stage or NB cases, especially those without MNA (Table 3). Importantly, 10 out of 12 Gal-3-positive NB cases were still disease-free at the time of last update, suggesting that both Gal-3 expression patterns described above (either in Schwannian or in neuroblastic cells) are associated with improved outcome. It is noteworthy that among Shimada unfavorable cases, two out of four (50%) Gal-3-positive patients were still alive, whereas survival declined to 13.2% in Gal-3-negative patients (Table 3).
NB cell differentiation associates with Gal-3 upregulation and nuclear relocalization
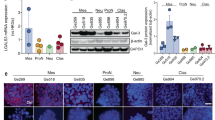
Gal-3 nuclear exclusion characterizes immature neuroblasts in several NB cases (Table 1, Figures 1c and d and Supplementary Table 1), consistent with data showing that MNA N-type (neuroblastic) NB cell lines express low levels of Gal-3 mostly localized in the cytoplasm34 (Figure 2d). Many MYCN-amplified cell lines undergo growth arrest, MYCN repression and neuronal differentiation upon treatment with the known neuronal differentiation factor all-trans retinoic acid (RA; Figures 2a–c).35, 36, 37 Along with these effects, RA also increased Gal-3 expression and promoted its nuclear accumulation in SK-N-BE2(c) and LAN-5 cell lines (Figures 2a–d).
Increased Gal-3 expression and nuclear localization are induced by RA. (a) Phase contrast microphotographs documenting the morphological differentiation and the induction of neurites by RA on the N-type SK-N-BE(2)c and LAN-5 NB cell lines. Along with this effect, RA also induced the well-known downregulation of MYCN, and a dose dependent (b) Gal-3 increase at the mRNA and protein level as evidenced by Q-PCR (c, upper panel) and immunoblotting (c, lower panel), respectively, in both N-type NB cell lines. (d) Immunofluorescence analysis of Gal-3 expression on fixed SK-N-BE(2)c and LAN-5 cells showed that RA treatment promoted Gal-3 localization to the cell nucleus
Nuclear Gal-3 induces cell growth inhibition and substrate adherence in NB cells
To assess whether Gal-3 expression is merely a marker of a less aggressive phenotype in NTs or an important biological determinant, we specifically overexpressed an myc-tagged version (Gal-3) or a nuclear localization restricted version (Nuc-Gal-3) of Gal-3 into SK-N-BE(2)c, LAN-5 and LAN-1 NB cell lines. Very importantly, Gal-3 overexpression impaired cell growth and colony-forming ability of multiple NB cell lines without inducing cell death (Figures 3a–d, Supplementary Figures S2A–C), and these effects were reproducibly magnified when Gal-3 expression was forced into the nucleus (see Nuc-Gal-3 effects in Figure 3 and Supplementary Figure S2). Free Gal-3 overexpression did not significantly alter the morphological aspect, nor did it affect RA-dependent differentiation in SK-N-BE(2)c cells (Figure 4A). In contrast, nuclear Gal-3 expression clearly affected the morphology of SK-N-BE(2)c cells, inducing a more fused, substrate-adherent phenotype and promoting a modest induction of neurites, which, however, failed to reach the same extent induced by RA (Figure 4A). Consistently, Gal-3 overexpression also induced increased substrate adherence in LAN-5 cells (Figure 4B), especially when its expression was forced into the cell nucleus.
Effects of Gal-3 overexpression on NB cell proliferation. (a) Forced expression of exogenous Gal-3 in the nucleus by fusion of WT Gal-3 to a triple nuclear localization signal (Nuc-Gal-3) impaired the colony-forming ability of LAN-5 cells (colony counts in a, lower panel). (b) Immunoblot showing the expression of the exogenous free Gal-3 (Gal-3) and nuclear Gal-3 (Nuc-Gal-3) in each individual cell clone. (c) Selected LAN-5 cell clones expressing either free Gal-3 or nuclear Gal-3 were used to assess the effect of Gal-3 on cell proliferation. LAN-5 cells expressing Gal-3 and cells expressing Nuc-Gal-3 showed a reduced proliferation rate compared with empty vector-transfected (pcDNA) or untransfected (Untr) counterparts. For each time point, data given represent the average (± S.D.s) of the cell counts obtained from the three pcDNA, the three Gal-3 or the three Nuc-Gal-3 transfected clones. Significant differences in cell proliferation at 24, 48 and 72 h were obtained in Gal-3- and Nuc-Gal-3 transfected clones compared with pcDNA-transfected ones (*P⩽0.05, **P<0.001, ***P<0.0001). Raw data for each LAN-5 cell clone are given in Supplementary Figure 3A.(d) BrdU incorporation assay showing the effect of pcDNA, Gal-3 and Nuc-Gal-3 transient transfection on DNA synthesis in SK-N-BE(2)c cells. A representative immunoblot of Gal-3 and Nuc-Gal-3 exogenous expression is given in the inset
Effects of Gal-3 overexpression on NB cell morphology and adhesion. (A) The immunofluorescence analysis of β 3-tubulin expression highlights the morphological changes induced by exogenous free Gal-3 or Nuc-Gal-3 expression or RA treatment compared with mock-transfected (pcDNA) SK-N-BE(2)c cells. (B) The nine LAN-5 cell clones used in Figure 3 were also used for adherence experiments. Detached cells were plated and after 2 h the percentage of adherent cells was determined. Results are expressed as the averages of the percentages (±S.D.s) obtained with the three pcDNA, Gal-3 or Nuc-Gal-3-transfected clones (***P<0.0001). Raw data for each LAN-5 cell clone are given in Supplementary Figure 3B
Discussion
Modulation of Gal-3 expression has been reported in different human malignancies during tumor progression,22, 23, 24, 25, 26, 27 and its analysis has acquired diagnostic relevance in the preoperative characterization of thyroid nodules, allowing a better selection of the candidates to surgery.38, 39, 40 The present study indicates that the routine immunohistochemical determination of Gal-3 expression may be a novel and clinically relevant prognostic marker for the stratification of patients with NT. Indeed, Gal-3 expression displays a positive predictive value on survival not only in the general cohort of NTs but also in older-age patients, advanced-stage tumors and in the NB histopathological subtype. Despite its strong association with Shimada favorable phenotype and with MYCN single-copy status, we also observed a trend for better survival rates in Gal-3-positive versus Gal-3-negative cases in the Shimada unfavorable group and MNA cases. Therefore, the characterization of Gal-3 expression in a larger series of patients and its statistical evaluation by a multiple regression analysis is mandatory, as so far very few biomolecular markers proved to be readily detectable with routine laboratory tests and effective for the clinical stratification of NTs.
Schwannian stroma represents a biologically relevant feature for NTs,12 but its nature is still controversial as inconsistent results have either supported or denied the origin of Schwannian and neuroblastic cells from a common tumor-initiating cell.13, 14, 15, 16 Furthermore, a possible ‘reactive nature’ of Schwannian cells populating NTs has been proposed.17 On the basis of Gal-3 expression, our study indicates that qualitatively and/or biologically different Schwannian stromal cells exist in NTs. Indeed, we observed nuclear and cytoplasmic Gal-3 expression in S-100-positive Schwannian cells of prognostically favorable stroma-rich tumors (such as GN and many GNBs) and six NB cases, five of which are still disease-free up until now. As Gal-3 expression is constitutive in normal Schwann cells and is markedly increased in Schwann cells of injured nervous fibers that acquire phagocytic and inflammatory cell features,41 we speculate that Gal-3 expression may identify reactive Schwannian stromal cells granting host advantage either contributing to a differentiating microenvironment and/or exhibiting phagocytic properties against tumor cells. In contrast, S-100-positive and Gal-3-negative Schwannian stromal cells characterized more aggressive NBs and GNBs, suggesting that these cells might be of a different nature and perhaps of tumor origin.
Despite differentiated ganglion cells occurring in NTs being invariably Gal-3-negative, we found Gal-3 expression predominantly in the cytoplasm of undifferentiated neuroblastic cells in six NB cases and one GNB case. Interestingly, we observed this particular pattern of Gal-3 expression in MNA N-type NB cells in vitro and showed that it is associated with enhanced resistance to chemotherapy.34 Therefore, the clinical relevance of this second pattern of Gal-3 expression should be interpreted with caution.
Altogether, our findings from NTs ex vivo analysis and in vitro experimental models pointed out that the Gal-3 expression is not only a marker of increased differentiation but likely has a pivotal role in neural cell differentiation. Indeed, its increased expression and nuclear localization is associated with RA-induced differentiation, and Gal-3 overexpression in N-type NB cell lines led to phenotypic differentiation, reduced proliferation and increased substrate adherence thus displaying ‘tumor-suppressive’ functions. Nuclear Gal-3 overexpression also impaired scratch wound repair in vitro (Supplementary Figure S3C). This observation definitely reaffirms the negative effects of nuclear Gal-3 on proliferation, and perhaps it suggests that Gal-3 nuclear versus cytoplasmic localization might regulate other cancer cell phenotypes, such as cell migration, which needs to be further and specifically investigated in the future. Although at the present we do not know how Gal-3 causes all these phenotypic changes, we speculate that it might do so via regulation of gene expression, consistent with the strongest effects being observed by its nuclear localization and in agreement with reports indicating a role for Gal-3 in gene transcription.42, 43
In conclusion, we identified the straightforward determination of Gal-3 expression by immunohistochemistry as a potential novel and important prognostic factor in NTs and provided insights into its biological role in neuroblastic cell differentiation and tumorigenesis.
Materials and Methods
Neuroblastic tumor samples
Primary NT samples from 128 children and teenagers (60 males, 68 females) affected by NTs and admitted at the Department of oncology, Alder Hey Children’s NHS Foundation Trust, the Department of Pediatrics and Infantile Neuropsychiatry, University La Sapienza and the Division of Oncology, Bambino Gesù Children’s Hospital were obtained at the time of first histopathological diagnosis. Patients were aged from 1 day to 180 months (median, 29 months). Histopathological categorization (see Supplementary Table 1) and histopathological grading according to the Shimada classification were carried out following INPC instructions.12, 44 Patients with NB and GNB were staged according to the International Neuroblastoma Staging System.45 As of April 2013, the median follow-up for the 128 patients was 59 months (range, 1–240), and further details on the status of each patient are given in Supplementary Table 1. The study was carried out according to the ethical guidelines of the declaration of Helsinki. A written informed consent was obtained from each enrolled patient or legal guardian. The study underwent ethical review and approval according to local institutional guidelines.
Molecular and immunohistochemical analysis
MYCN gene copy number was determined as described46, 47 in 125 out of the 128 tumor samples, and MYCN amplification was detected in 31 cases (24%). Tissue sections from formalin-fixed and paraffin-embedded tissue specimens were used for conventional histo-morphological evaluation and immunophenotypical analysis. Purified rat mAb HRP-conjugated to Gal-3 (Space Import Export, Milan Italy; 5 μg/ml) and commercially acquired mouse mAb to S-100 (DakoCytomation, Glostrup, Denmark) were used in a direct immunoperoxidase assay, as previously reported.48, 49
Cell lines and culture conditions
SK-N-BE(2)c cells were acquired from European Collection of Cell Cultures (Porton Down, UK; http://www.phe-culturecollections.org.uk/collections/ecacc.aspx); LAN-5 cells were from Deutsche Sammlung Von Mikroorganismen und Zellkulturen (Braunschweig, Germany; www.dsmz.de). LAN-1 cells were a kind gift of Dr. Nicole Gross, Department of Pediatrics, University Hospital, Lausanne, Switzerland. All cells were grown in standard conditions and validated by genetic determination of MYCN amplification or other genetic aberrations.
Constructs and transfections
The Nuc-Gal-3 vector was obtained by fusing the PCR-amplified human Gal-3 coding sequence to a triple nuclear localization signal in the pcDNA3.1(-)/Myc-His A vector (Invitrogen, San Diego, CA, USA). Other Gal-3 constructs and transfection conditions were previously described.34
RNA and protein analysis
Total RNA extraction was carried out with trizol reagent (Invitrogen), and quantitative reverse transcription-PCR (Q-RT–PCR) was performed as described.34
Total protein extracts were obtained and separated as described.34 The following MoAbs were used: mouse anti-MYCN and mouse anti-β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and rat anti-Gal-3 purified monoclonal antibody (Space Import & Export). Immunoreactive bands were visualized by enhanced chemoluminescence (Perkin Elmer, Waltham, MA, USA).
Immunofluorescence analysis, apoptosis assays and adherence test
Indirect immunofluorescence was used to study the expression of Gal-3 and β3-tubulin in cell lines and transfectants.37 Cells were fixed in 4% formaldehyde/PBS for 10 min, permeabilized in 0.25% triton/PBS for 10 min and incubated overnight with primary Abs, followed by secondary FITC-conjugated species-specific antisera (Invitrogen).
Apoptosis assays, including analysis of nuclear morphology of Hoechst 33258-stained cells and detection of the p85 cleaved fragment of the PARP protein (p85PARP) by immunofluorescence, were performed as described.50 For each apoptosis assay, at least 200 cells/sample were counted in duplicate experiments. Each experiment was performed at least three times. Statistical analysis was performed by a standard two-tailed Student’s t-test.
Primary and secondary Abs further than the following are described in :37 MoAb anti-β3-tubulin (Merck Millipore, Darmstadt, Germany). For adherence assay, 2 h after cell seeding, the medium was removed and the percentage of adherent cells was determined using Trypan blue exclusion test.
Colony formation and cell proliferation assays
For colony formation assay, Gal-3-transfected and untrasfected LAN-5 and LAN-1 cells were exposed to 400 μg/ml G418, and colonies were counted after 21 days. Individual groups of 15 or more cells were counted as ‘colonies’. For cell proliferation assay, either cell number was measured by trypan blue exclusion test or cell proliferation was assayed by the 5-Bromo-2′-deoxy-uridine Labeling and Detection Kit I (Roche Diagnostics, Indianapolis, IN, USA), as recommended by the manufacturer. At least 200 cells/sample were counted in triplicate experiments.
Statistical analysis
Frequencies of Gal-3 expression versus known prognostic factors were cross-tabulated, using two-tailed Fisher’s Exact Test or Pearson’s χ2-test to determine the significance of the associations. Five-year OS was evaluated by the Kaplan–Meier method, and the differences between subgroups (of patients) were tested using log-rank test. P-values <0.05 were considered statistically significant. The analyses were carried out using the software package SPSS 11.0 for Windows (SPSS Inc., Chicago, IL, USA).
Abbreviations
- NTs:
-
neuroblastic tumors
- NB:
-
neuroblastoma
- INRG:
-
International Neuroblastoma Risk Group
- INPC:
-
International Neuroblastoma Pathology Committee
- GNB:
-
ganglioneuroblastoma
- GN:
-
ganglioneuroma
- MKI:
-
mitosis-karyorrhexis index
- Gal-3:
-
galectin-3
- INSS:
-
International Neuroblastoma Staging System
References
Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM . Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J 1997; 16: 2985–2995.
Bordow SB, Norris MD, Haber PS, Marshall GM, Haber M . Prognostic significance of MYCN oncogene expression in childhood neuroblastoma. J Clin Oncol 1998; 16: 3286–3294.
Bagatell R, Beck-Popovic M, London WB, Zhang Y, Pearson AD, Matthay KK et al. Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: a report from the International Neuroblastoma Risk Group database. J Clin Oncol 2009; 27: 365–370.
Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013; 45: 279–284.
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol 2009; 27: 289–297.
Vermeulen J, De Preter K, Mestdagh P, Laureys G, Speleman F, Vandesompele J . Predicting outcomes for children with neuroblastoma. Discov Med 2010; 10: 29–36.
Light JE, Koyama H, Minturn JE, Ho R, Simpson AM, Iyer R et al. Clinical significance of NTRK family gene expression in neuroblastomas. Pediatr Blood Cancer 2012; 59: 226–232.
Vermeulen J, De Preter K, Naranjo A, Vercruysse L, Van Roy N, Hellemans J et al. Predicting outcomes for children with neuroblastoma using a multigene-expression signature: a retrospective SIOPEN/COG/GPOH study. Lancet Oncol 2009; 10: 663–671.
De Preter K, Mestdagh P, Vermeulen J, Zeka F, Naranjo A, Bray I et al. miRNA expression profiling enables risk stratification in archived and fresh neuroblastoma tumor samples. Clin Cancer Res 2011; 17: 7684–7692.
Ohira M, Nakagawara A . Global genomic and RNA profiles for novel risk stratification of neuroblastoma. Cancer Sci 2010; 101: 2295–2301.
Shimada H, Umehara S, Monobe Y, Hachitanda Y, Nakagawa A, Goto S et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children's Cancer Group. Cancer 2001; 92: 2451–2461.
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 1999; 86: 364–372.
Mora J, Cheung NK, Juan G, Illei P, Cheung I, Akram M et al. Neuroblastic and Schwannian stromal cells of neuroblastoma are derived from a tumoral progenitor cell. Cancer Res 2001; 61: 6892–6898.
Bourdeaut F, Ribeiro A, Paris R, Pierron G, Couturier J, Peuchmaur M et al. In neuroblastic tumours, Schwann cells do not harbour the genetic alterations of neuroblasts but may nevertheless share the same clonal origin. Oncogene 2008; 27: 3066–3071.
Acosta S, Lavarino C, Paris R, Garcia I, de Torres C, Rodriguez E et al. Comprehensive characterization of neuroblastoma cell line subtypes reveals bilineage potential similar to neural crest stem cells. BMC Dev Biol 2009; 9: 12.
Acosta S, Mayol G, Rodriguez E, Lavarino C, de Preter K, Kumps C et al. Identification of tumoral glial precursor cells in neuroblastoma. Cancer Lett 2011; 312: 73–81.
Du W, Hozumi N, Sakamoto M, Hata J, Yamada T . Reconstitution of Schwannian stroma in neuroblastomas using human bone marrow stromal cells. Am J Pathol 2008; 173: 1153–1164.
Dumic J, Dabelic S, Flogel M . Galectin-3: an open-ended story. Biochim Biophys Acta 2006; 1760: 616–635.
Califice S, Castronovo V, Bracke M, van den Brule F . Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 versus tumor promotion of cytoplasmic galectin-3. Oncogene 2004; 23: 7527–7536.
Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T et al. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res 2006; 66: 3114–3119.
Yu F, Finley RL Jr., Raz A, Kim HR . Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem 2002; 277: 15819–15827.
Canesin G, Gonzalez-Peramato P, Palou J, Urrutia M, Cordon-Cardo C, Sanchez-Carbayo M . Galectin-3 expression is associated with bladder cancer progression and clinical outcome. Tumour Biol 2010; 31: 277–285.
Endo K, Kohnoe S, Tsujita E, Watanabe A, Nakashima H, Baba H et al. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res 2005; 25: 3117–3121.
Idikio H . Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int J Oncol 1998; 12: 1287–1290.
Kim MK, Sung CO, Do IG, Jeon HK, Song TJ, Park HS et al. Overexpression of Galectin-3 and its clinical significance in ovarian carcinoma. Int J Clin Oncol 2011; 16: 352–358.
Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V et al. Decreased galectin-3 expression in prostate cancer. Prostate 2000; 44: 118–123.
Shimamura T, Sakamoto M, Ino Y, Shimada K, Kosuge T, Sato Y et al. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res 2002; 8: 2570–2575.
Narciso MS, Mietto Bde S, Marques SA, Soares CP, Mermelstein Cdos S, El-Cheikh MC et al. Sciatic nerve regeneration is accelerated in galectin-3 knockout mice. Exp Neurol 2009; 217: 7–15.
Pesheva P, Kuklinski S, Biersack HJ, Probstmeier R . Nerve growth factor-mediated expression of galectin-3 in mouse dorsal root ganglion neurons. Neurosci Lett 2000; 293: 37–40.
Gustavsson P, Linsmeier CE, Leffler H, Kanje M . Galectin-3 inhibits Schwann cell proliferation in cultured sciatic nerve. Neuroreport 2007; 18: 669–673.
Pesheva P, Kuklinski S, Schmitz B, Probstmeier R . Galectin-3 promotes neural cell adhesion and neurite growth. J Neurosci Res 1998; 54: 639–654.
Binh NH, Satoh K, Kobayashi K, Takamatsu M, Hatano Y, Hirata A et al. Galectin-3 in preneoplastic lesions of glioma. J Neurooncol 2013; 111: 123–132.
Petroni M, Veschi V, Gulino A, Giannini G . Molecular mechanisms of MYCN-dependent apoptosis and the MDM2-p53 pathway: an Achille's heel to be exploited for the therapy of MYCN-amplified neuroblastoma. Front Oncol 2012; 2: 141.
Veschi V, Petroni M, Cardinali B, Dominici C, Screpanti I, Frati L et al. Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells. PloS one 2012; 7: e49139.
Giannini G, Dawson MI, Zhang X, Thiele CJ . Activation of three distinct RXR/RAR heterodimers induces growth arrest and differentiation of neuroblastoma cells. J Biol Chem 1997; 272: 26693–26701.
Giannini G, Di Marcotullio L, Ristori E, Zani M, Crescenzi M, Scarpa S et al. HMGI(Y) and HMGI-C genes are expressed in neuroblastoma cell lines and tumors and affect retinoic acid responsiveness. Cancer Res 1999; 59: 2484–2492.
Giannini G, Kim CJ, Marcotullio LD, Manfioletti G, Cardinali B, Cerignoli F et al. Expression of the HMGI(Y) gene products in human neuroblastic tumours correlates with differentiation status. Br J Cancer 2000; 83: 1503–1509.
Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A et al. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001; 357: 1644–1650.
Bartolazzi A, Orlandi F, Saggiorato E, Volante M, Arecco F, Rossetto R et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol 2008; 9: 543–549.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19: 1167–1214.
Reichert F, Saada A, Rotshenker S . Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci 1994; 14: 3231–3245.
Song S, Byrd JC, Mazurek N, Liu K, Koo JS, Bresalier RS . Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology 2005; 129: 1581–1591.
Braeuer RR, Zigler M, Kamiya T, Dobroff AS, Huang L, Choi W et al. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res 2012; 72: 5757–5766.
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B . Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer 1999; 86: 349–363.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993; 11: 1466–1477.
Dominici C, Negroni A, Romeo A, Castello MA, Clerico A, Scopinaro M et al. Association of near-diploid DNA content and N-myc amplification in neuroblastomas. Clin Exp Metastasis 1989; 7: 201–211.
Giannini G, Cerignoli F, Mellone M, Massimi I, Ambrosi C, Rinaldi C et al. High mobility group A1 is a molecular target for MYCN in human neuroblastoma. Cancer Res 2005; 65: 8308–8316.
Bartolazzi A, Bellotti C, Sciacchitano S . Methodology and technical requirements of the galectin-3 test for the preoperative characterization of thyroid nodules. Appl Immunohistochem Mol Morphol 2012; 20: 2–7.
Martegani MP, Del Prete F, Gasbarri A, Natali PG, Bartolazzi A . Structural variability of CD44v molecules and reliability of immunodetection of CD44 isoforms using mAbs specific for CD44 variant exon products. Am J Pathol 1999; 154: 291–300.
Petroni M, Veschi V, Prodosmo A, Rinaldo C, Massimi I, Carbonari M et al. MYCN sensitizes human neuroblastoma to apoptosis by HIPK2 activation through a DNA damage response. Mol Cancer Res 2011; 9: 67–77.
Acknowledgements
We are grateful to Dr. Carol J Thiele for careful reading of the manuscript and helpful suggestions and to Kristel Bejo for technical help in performing immunohistochemical and histological procedures. This work was partially supported by grants from Associazione Italiana per la Ricerca sul Cancro to GG (IG12116) and AG, AIRC 5XMILLE, MIUR FIRB and PRIN projects, Ministry of Health, EU HEALING grant number 238186, IIT (Italian Institute of Technology), ASI (Agenzia Spaziale Italiana) and Pasteur Institute/Cenci Bolognetti Foundation. MP is a recipient of a FIRC fellowship David Raffaelli.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Verkhratsky
Supplementary Information accompanies this paper on Cell Death and Disease website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Veschi, V., Petroni, M., Bartolazzi, A. et al. Galectin-3 is a marker of favorable prognosis and a biologically relevant molecule in neuroblastic tumors. Cell Death Dis 5, e1100 (2014). https://doi.org/10.1038/cddis.2014.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2014.68
Keywords
This article is cited by
-
Targeting galectin-driven regulatory circuits in cancer and fibrosis
Nature Reviews Drug Discovery (2023)
-
MRE11 inhibition highlights a replication stress-dependent vulnerability of MYCN-driven tumors
Cell Death & Disease (2018)
-
PARP inhibitors enhance replication stress and cause mitotic catastrophe in MYCN-dependent neuroblastoma
Oncogene (2017)