Abstract
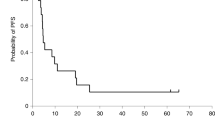
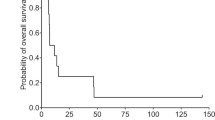
The aim of this phase I clinical trial was to assess the feasibility and safety of intratumoral administration of a first-generation adenoviral vector encoding herpes simplex virus thymidine kinase (HSV-TK) gene (Ad.TK) followed by systemic ganciclovir to patients with advanced hepatocellular carcinoma (HCC). Secondarily, we have analyzed its antitumor effect. Ten patients were enrolled in five dose-level cohorts that received from 1010 to 2 × 1012 viral particles (vp). Ad.TK was injected intratumorally and patients received up to three doses at 30-day intervals. Positron emission tomography was used to monitor TK gene expression. Ad.TK injection was feasible in 100% of cases. Treatment was well tolerated and dose-limiting toxicity was not achieved. Cumulative toxicity was not observed. Hepatic toxicity was absent even in cirrhotic patients. Fever, flu-like syndrome, pain at the injection site and pancytopenia were the most common side effects. No partial responses were observed and 60% of patients showed tumor stabilization of the injected lesion. Importantly, two patients who received the highest dose showed signs of intratumoral necrosis by imaging procedures. One of them achieved a sustained stabilization and survived for 26 months. In conclusion, Ad.TK can be safely administered by intratumoral injection to patients with HCC up to 2 × 1012 vp per patient.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P . Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108.
El-Serag HB, Mason AC . Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999; 340: 745–750.
El-Serag HB, Marrero JA, Rudolph L, Reddy KR . Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134: 1752–1763.
Bruix J, Sherman M . Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236.
Llovet JM, Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008; 100: 698–711.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
Avila MA, Berasain C, Sangro B, Prieto J . New therapies for hepatocellular carcinoma. Oncogene 2006; 25: 3866–3884.
Mohr L, Geissler M, Blum HE . Gene therapy for malignant liver disease. Expert Opin Biol Ther 2002; 2: 163–175.
Fillat C, Carrio M, Cascante A, Sangro B . Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther 2003; 3: 13–26.
Shen Y, Nemunaitis J . Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther 2006; 13: 975–992.
Vassaux G, Martin-Duque P . Use of suicide genes for cancer gene therapy: study of the different approaches. Expert Opin Biol Ther 2004; 4: 519–530.
Matthews T, Boehme R . Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis 1998; 10 (Suppl 3): S490–S494.
Moolten FL . Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res 1986; 46: 5276–5281.
Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 1993; 53: 5274–5283.
Kianmanesh AR, Perrin H, Panis Y, Fabre M, Nagy HJ, Houssin D et al. A “distant” bystander effect of suicide gene therapy: regression of nontransduced tumors together with a distant transduced tumor. Hum Gene Ther 1997; 8: 1807–1814.
Kuriyama S, Kikukawa M, Masui K, Okuda H, Nakatani T, Akahane T et al. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer 1999; 83: 374–380.
Qian C, Idoate M, Bilbao R, Sangro B, Bruña O, Vázquez J et al. Gene transfer and therapy with adenoviral vector in rats with diethylnitrosamine-induced hepatocellular carcinoma. Hum Gene Ther 1997; 8: 349–358.
Bilbao R, Gérolami R, Bralet MP, Qian C, Tran PL, Tennant B et al. Transduction efficacy, antitumoral effect, and toxicity of adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir therapy of hepatocellular carcinoma: the woodchuck animal model. Cancer Gene Ther 2000; 7: 657–662.
Qian C, Bilbao R, Bruna O, Prieto J . Induction of sensitivity to ganciclovir in human hepatocellular carcinoma cells by adenovirus-mediated gene transfer of herpes simplex virus thymidine kinase. Hepatology 1995; 22: 118–123.
Herraiz M, Beraza N, Solano A, Sangro B, Montoya J, Qian C et al. Liver failure caused by herpes simplex virus thymidine kinase plus ganciclovir therapy is associated with mitochondrial dysfunction and mitochondrial DNA depletion. Hum Gene Ther 2003; 14: 463–472.
Su H, Lu R, Chang JC, Kan YW . Tissue-specific expression of herpes simplex virus thymidine kinase gene delivered by adeno-associated virus inhibits the growth of human hepatocellular carcinoma in athymic mice. Proc Natl Acad Sci USA 1997; 94: 13891–13896.
Penuelas I, Mazzolini G, Boán JF, Sangro B, Martí-Climent J, Ruiz M et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology 2005; 128: 1787–1795.
WHO: Handbook of Reporting Results of Cancer Treatment 1979. Publication No. 48. WHO: Geneva, Switzerland.
National Cancer Institute: Guidelines for the Reporting of Adverse Drug Reactions. National Cancer Institute: Bethesda, MD, 1990, pp 1–80.
Karch FE, Lasagna L . Adverse drug reactions. A critical review. JAMA 1975; 234: 1236–1241.
Penuelas I, Boán JF, Martí-Climent JM, Barajas MA, Narvaiza I, Satyamurthy N et al. A fully automated one pot synthesis of 9-(4-[18F]fluoro-3-hydroxymethylbutyl) guanine for gene therapy studies. Mol Imaging Biol 2002; 4: 415–424.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–430.
Wilson JM . Adenoviruses as gene-delivery vehicles. N Engl J Med 1996; 334: 1185–1187.
Drozdzik M, Qian C, Xie X, Peng D, Bilbao R, Mazzolini G et al. Combined gene therapy with suicide gene and interleukin-12 is more efficient than therapy with one gene alone in a murine model of hepatocellular carcinoma. J Hepatol 2000; 32: 279–286.
Li N, Zhou J, Weng D, Zhang C, Li L, Wang B et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2007; 13: 5847–5854.
Sung MW, Yeh HC, Thung SN, Schwartz ME, Mandeli JP, Chen SH et al. Intratumoral adenovirus-mediated suicide gene transfer for hepatic metastases from colorectal adenocarcinoma: results of a phase I clinical trial. Mol Ther 2001; 4: 182–191.
Penuelas I, Haberkorn U, Yaghoubi S, Gambhir SS . Gene therapy imaging in patients for oncological applications. Eur J Nucl Med Mol Imaging 2005; 32 (Suppl 2): S384–S403.
Sangro B, Qian C, Ruiz J, Prieto J . Tracing transgene expression in cancer gene therapy: a requirement for rational progress in the field. Mol Imaging Biol 2002; 4: 27–33.
Ido A, Uto H, Moriuchi A, Nagata K, Onaga Y, Onaga M et al. Gene therapy targeting for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by a hypoxia-inducible enhancer linked to a human alpha-fetoprotein promoter. Cancer Res 2001; 61: 3016–3021.
Teh BS, Aguilar-Cordova E, Kernen K, Chou CC, Shalev M, Vlachaki MT et al. Phase I/II trial evaluating combined radiotherapy and in situ gene therapy with or without hormonal therapy in the treatment of prostate cancer--a preliminary report. Int J Radiat Oncol Biol Phys 2001; 51: 605–613.
Stefani AL, Barzon L, Castagliuolo I, Guido M, Pacenti M, Parolin C et al. Systemic efficacy of combined suicide/cytokine gene therapy in a murine model of hepatocellular carcinoma. J Hepatol 2005; 42: 728–735.
Torimura T, Ueno T, Kin M, Taniguchi E, Nakamura T, Inoue K et al. Gene transfer of kringle 1-5 suppresses tumor development and improves prognosis of mice with hepatocellular carcinoma. Gastroenterology 2006; 130: 1301–1310.
Shinozaki K, Suominen E, Carrick F, Sauter B, Kähäri VM, Lieber A et al. Efficient infection of tumor endothelial cells by a capsid-modified adenovirus. Gene Ther 2006; 13: 52–59.
Acknowledgements
CIBERehd is funded by the Instituto de Salud Carlos III. This work has been funded in part by Fundación Areces and Accion Transversal contra el Cancer. We thank Maria Eugenia Cornet, Maria del Mar Municio, Viñas Andrés and Elena del Corral for their work in trial monitoring.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sangro, B., Mazzolini, G., Ruiz, M. et al. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther 17, 837–843 (2010). https://doi.org/10.1038/cgt.2010.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.40
Keywords
This article is cited by
-
Peptide-guided JC polyomavirus-like particles specifically target bladder cancer cells for gene therapy
Scientific Reports (2021)
-
Regenerative therapy for spinal cord injury using iPSC technology
Inflammation and Regeneration (2020)
-
Demonstration of anti-tumour bystander killing with prodrug-preloaded suicide gene-engineered tumour cells: a potential improvement for cancer therapeutics
Cancer Cell International (2020)
-
The impact of γ-irradiation on the induction of bystander killing by genetically engineered ovarian tumor cells: implications for clinical use
Cancer Cell International (2017)
-
Enzyme/Prodrug Systems for Cancer Gene Therapy
Current Pharmacology Reports (2016)