Abstract
The tumor microenvironment (TME) plays a prominent role in the growth of tumor cells. As the major inflammatory component of the TME, M2d macrophages are educated by the TME such that they adopt an immunosuppressive role that promotes tumor metastasis and progression. Fra-1 forms activator protein-1 heterodimers with Jun partners and drives gene transcription. Fra-1 is thought to drastically induce tumorigenesis and progression. However, the functional role of Fra-1 in the generation of M2d macrophages is poorly understood to date. Here, we demonstrate that 4T1 mammary carcinoma cells, when co-cultured with RAW264.7 macrophage cells, skew the RAW264.7 macrophage cell differentiation into M2d macrophages. The 4T1 cells stimulate de novo overexpression of Fra-1 in RAW264.7 cells, and then Fra-1 binds to the interleukin 6 (IL-6) promoter to increase the production of the cytokine IL-6 in RAW264.7 cells. IL-6 acts in an autocrine fashion to skew RAW264.7 macrophage cell differentiation into M2d macrophages. These findings open new insights into how to reverse M2d macrophage-induced immune tolerance to improve the efficacy of immunotherapeutic approaches.
Similar content being viewed by others
Introduction
Tumor development is a consequence of the provision of a fertile environment (the soil) in which compatible tumor cells (the seed) can proliferate; therefore, the tumor microenvironment (TME) plays an important role in the growth of tumor cells. The TME can modify the neoplastic properties of tumor cells 1. Inflammation is a critical component of tumor progression; many cancers arise at sites of infection, chronic irritation and inflammation 2, 3. Currently, it is clear that the TME, which is largely infiltrated by inflammatory cells, is an indispensable participant in the progression and metastasis of tumors 3. Macrophages are a major component of the leukocyte infiltrate of tumors 2, 4. As the major inflammatory component of the stroma of many tumors, tumor-associated macrophages (TAMs) can affect different aspects of the neoplastic tissue 1, 5. Recent experimental studies indicate that TAMs play a detrimental protumor role in the initiation of tumorigenesis, promotion of angiogenesis and lymphangiogenesis, growth, invasion and metastasis of tumor cells and the suppression of adaptive immunity 1, 5, 6, 7, 8. Clinical studies have shown that a high density of these TAMs correlates with a poor prognosis in over 80% of studies published, such as those in breast, prostate, cervical and ovarian cancers 9. TAMs thereby appear to be a potential drug target for antitumor therapy. Luo Y et al. 10 reported that decreasing the number of TAMs in the tumor stroma effectively altered the TME involved in tumor angiogenesis and progression, thereby markedly suppressing tumor growth and metastasis.
Macrophages are plastic cells and can be divided into two subsets, classically activated macrophages (or M1) and alternatively activated macrophages (or M2), on the basis of their ability to produce interleukin 12 (IL-12) and IL-10, respectively 11. M2 is further subdivided into M2a, M2b and M2c as elicited by IL-4 or IL-13, IL-1R ligands or exposure to immune complexes plus LPS and IL-10, respectively 11. M2 macrophages function in tuning the inflammatory responses and adaptive Th1 immunity, scavenging debris and promoting angiogenesis, tissue remodeling and repair, all of which are apparently different from the functions of M1 cells 12. TAMs show similar functions as M2s and exhibit an IL-10high IL-12low phenotype. A Mantovani et al. 12 demonstrated that TAMs are a polarized M2 macrophage population. In a recent study, Duluc et al. 13 reported that TAMs represent a novel M2 subset, which was named M2d (compared to M2a-c).
M2d macrophages are derived from circulating monocytes that are recruited to the tumor site by chemotactic factors, such as CCL2, monocyte colony-stimulating factor (M-CSF) and VEGF 8, 14. It is thought that the TME induces macrophages to differentiate toward M2d macrophages. Currently, the mechanism that programs the M2d macrophage phenotype remains unknown. Duluc et al. 13 identified leukemia inhibitory factor and IL-6 as factors present in the TME that act in concert with M-CSF to induce TAM-like cell generation.
IL-6 is a pleiotropic cytokine that mediates a plethora of physiological functions, including gene activation, cell proliferation and differentiation 15. IL-6 binds to a heterodimeric receptor, which contains the ligand-binding IL-6Rα chain and the common cytokine receptor signal-transducing subunit, gp130 15. Normally, IL-6 expression is tightly regulated, but an elevated expression of IL-6 has been detected in multiple epithelial tumors 15. IL-6 has also been implicated in various tumor progression steps, and IL-6 can promote the growth of numerous tumor cell lines and increase their resistance to apoptosis 16, 17. IL-6 also increases the resistance of breast cancer cells to chemotherapeutic treatment 16; moreover, a partial reduction of the actions or levels of IL-6 can restore the sensitivity of myeloma cells to chemotherapeutic drugs 17, 18. High serum IL-6 levels are a marker of poor prognosis in breast cancer 19 and myeloma patients 20. This affirms the possibility that IL-6 may play a critical role in cancer development and progression.
As mentioned above, the function of M2d macrophages is closely related to IL-6 in the promotion of tumor progression. IL-6 23, 24, IL-10 21 and VEGF 22, 23, 24 are present in the TME and might contribute to M2d macrophage accumulation by preventing monocytes from differentiating into DCs.
The IL-6 promoter is composed of a variety of overlapping regulatory elements 25, 26. Binding sites for the inducible transcription factors NF-κB, NF-IL-6, cAMP-responsive element-binding protein and activator protein-1 (AP-1) are essential for induction of the IL-6 gene 29, 30, 31. The activation of p50, p65, AP-1, JunD and Fra-1 in androgen-independent prostate cancer cells results in deregulated IL-6 expression 27. Although a variety of regulatory elements are involved in the regulation of IL-6 gene expression, how IL-6 is deregulated in M2d macrophages remains unknown.
Activator protein-1 is a transcription factor that consists of the JUN, FOS, ATF (activating transcription factor) and MAF (musculoaponeurotic fibrosarcoma) protein families, which recognize either 12-O-tetradecanoylphorbol-13-acetate-response elements (5′-TGAG/CTCA-3′) or cAMP-response elements (5′-TGACGTCA-3′) to regulate gene transcription 28. FOS family members are not able to form homodimers, but instead form heterodimers with JUN partners, giving rise to various transactivating or transrepressing complexes with different biochemical properties 29. Several studies have reported that AP-1 is involved in cellular proliferation, transformation and death 30, 31, and furthermore, reported an important role of Fra-1, which is one of the members of the FOS family, in tumor progression and metastasis 32.
In this study, we determined the molecular mechanisms underlying the transcriptional activation of the IL-6 promoter and deregulated IL-6 gene expression in M2d macrophages. Here, we provide evidence that Fra-1 gene expression is upregulated in M2d macrophages. Fra-1 upregulates the activation of IL-6 expression via activation of the IL-6 promoter in RAW264.7 cells and thereby promotes M2d differentiation in the co-culture of RAW264.7 cells and 4T1 cells. Furthermore, we show that knockdown of Fra-1 downregulates IL-6 expression at the mRNA and protein levels and leads to the inhibition of M2d differentiation.
Results
4T1 cells can skew RAW264.7 cell differentiation into M2d macrophages
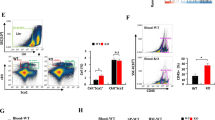
It is thought that the TME induces macrophages to polarize toward M2d macrophages, and that the contact between tumor cells and macrophages plays an important role in tumor development and progression. Therefore, we hypothesized that the contacts between macrophages and tumor cells could skew the macrophage differentiation into M2d macrophages in vitro. To test the hypothesis, we performed co-culture experiments with RAW264.7 cells and 4T1 cells to detect the percentage of M2d macrophages (F4/80+CD206+) in the co-culture at different time points (24 h, 48 h, 72 h and 96 h). As shown in Figure 1A, the percentage of M2d macrophages in both pure RAW264.7 cells and pure 4T1 cells was small. However, the percentage of M2d macrophages was significantly larger in co-cultured cells compared with that in pure RAW264.7 cells at all time points; moreover, the percentage of M2d macrophages in the co-culture experiment increased in a time-dependent manner. Similar results were obtained when we performed inducing experiments, in which RAW264.7 cells were incubated without (WT-RAWs) or with (SN-RAWs) the supernatant of 4T1 cells for 72 h; the resulting percentage of CD206+ cells was significantly larger in SN-RAWs compared with that in the matched WT-RAWs (Figure 1B). M2d macrophages express high levels of the mannose receptor (CD206) 12, and our results suggest that 4T1 can skew RAW264.7 differentiation toward M2d macrophages.
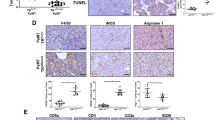
4T1 cells skew RAW264.7 cell differentiation toward M2d macrophages. (A) Flow cytometric analysis of the percentages of M2d macrophages in pure RAW264.7 cells (pure RAWs), pure 4T1 cells (pure 4T1s) or RAWs + 4T1s co-culture (1:4 RAWs: 4T1s). The percentages of M2d macrophages in the co-culture were detected at different time points as indicated. F4/80 is a specific surface marker of macrophages, and M2d macrophages were identified by positive staining with anti-F4/80-PE antibody and anti-CD206-FITC antibody. The percentages of the double-positive cells are shown. (B) RAW264.7 cells were incubated without (WT-RAWs) or with (SN-RAWs) the supernatant of 4T1 cells for 72 h, and flow cytometric analysis was performed to analyze the percentages of CD206+ cells among SN-RAWs versus WT-RAWs. The percentages of the CD206+ cells are shown. (C) Co-cultured RAW264.7 and 4T1 cells (at 72 h) were stained with PE-conjugated anti-mouse F4/80; C-RAWs cells (F4/80+ cells) and C-4T1s (F4/80− cells) were sorted by a FACS Aria sorter, as indicated, and the purities were routinely 94.3% and 99.7%, respectively. (D) Total RNA was prepared from mouse peritoneal macrophages (MΦs), wild-type RAW264.7 cells (WT-RAWs) and co-cultured RAW264.7 cells (C-RAWs). RT-PCR analysis was carried out using the CD80-, CD86-, MHC-II-, IL-15- or β-actin-specific primers. The results are representative of three independent experiments. (E) M1 or M2 phenotype genes expression in WT-RAWs versus C-RAWs; WT-RAWs and C-RAWs were treated with LPS (100 ng/ml) or rabbit anti-mouse IL-6 (500 ng/ml) as indicated, and total RNA was analyzed by real-time PCR in triplicates for the mRNA expression of IL-10, IL-12p35, CCL2, CCL22, TNF-α, TGF-β, iNOS and Arg1. The results were normalized to the expression of the housekeeping gene β-actin. The data are expressed as fold changes in mRNA expression with respect to the wild-type cells.
Similar to M2s, M2d macrophages express low levels of proinflammatory cytokines, such as IL-12 and IL-15 7, and are poor antigen-presenting cells 12. After 72 h of co-culture, RAW264.7 cells and 4T1 cells were isolated from co-culture and referred to as co-cultured RAW264.7 cells (C-RAWs) and co-cultured 4T1 cells (C-4T1s) (Figure 1C). Then, we compared the levels of mRNA expression of the co-stimulatory molecules CD80 and CD86 and of MHC-II and IL-15 for mouse peritoneal macrophages (MΦs), wild-type RAW264.7 cells (WT-RAWs) and C-RAWs. As shown in Figure 1D, C-RAWs expressed lower levels of CD80, CD86, MHC-II and IL-15 mRNA than Mφs and WT-RAWs. Furthermore, Figure 1E shows the defective expression of M1 phenotype genes (IL-12p35, TNF-α and iNOS) and high expression levels of the M2 phenotype genes (IL-10, CCL2, CCL22 and Arg1) in LPS-activated C-RAWs. These data show that the C-RAWs exhibit similar phenotypic and functional characteristics as M2d macrophages.
Taken together, these data demonstrate that 4T1 cells can skew RAW264.7 cell differentiation toward M2d macrophages.
IL-6 produced by C-RAWs induces M2d macrophage generation via an autocrine pathway
As shown in Figure 1B, the supernatant of 4T1 cells can skew RAW264.7 cell differentiation into M2d macrophages, but which cytokines in the supernatant play a critical role in the differentiation is unknown. As described previously, IL-6 has been implicated in the progression of various tumors and also increases the resistance of breast cancer cells to chemotherapy 16. High serum IL-6 levels are a marker of poor prognosis in breast cancer. Therefore, it is of interest to ask whether IL-6 might promote tumor progression via inducing M2d macrophage generation. We analyzed IL-6 protein levels in culture supernatants obtained from co-cultures, pure RAW264.7 cells and pure 4T1 cells at 24, 48, 72 and 96 h time points. As expected, IL-6-specific ELISA analysis revealed that the co-cultured cells secreted drastically higher levels of IL-6 compared with pure RAW264.7 cells and pure 4T1 cells at all time points (Figure 2A). This suggests that IL-6 is a main factor involved in M2d macrophage generation. To verify this, a neutralizing anti-mouse IL-6 antibody was added in the co-culture, and then we analyzed the percentage of M2d macrophages (F4/80+CD206+) in the co-culture and the expression of special phenotype genes at 72 h. The percentage of M2d macrophages decreased (Figure 2B) compared with that of controls. As shown in Figure 1E, C-RAWs treated with anti-IL-6 antibody expressed higher levels of IL-12p35 and TNF-α mRNA in response to LPS compared with controls. In contrast, anti-IL-6 antibody in the co-culture resulted in an appreciable decrease of IL-10, CCL2, CCL22 and Arg1 mRNA expression in C-RAWs treated with LPS. These data indicate that IL-6 in the supernatant of co-cultured cells induces the generation of M2d macrophages.
IL-6 produced by C-RAWs plays a major role in inducing M2d macrophage generation. (A) IL-6 ELISA on the cell-free supernatant of pure RAW264.7 cells (pure RAWs), pure 4T1 cells (pure 4T1s) or RAWs + 4T1s co-culture (1:4 RAWs:4T1s) at the indicated time points. The results are expressed in ng/ml. Columns are representative of the three independent experiments carried out in quadruplicate. Bars, means±SD. P < 0.01 (by one-way ANOVA). (B) RAWs + 4T1s co-culture was maintained in CM with neutralizing rabbit anti-mouse IL-6 (500 ng/ml) or isotype IgG antibody (500 ng/ml) (added at hours 0, 24 and 48). The percentage of M2d macrophages in the co-culture was detected at 72 h. The percentages of the double-positive cells are shown. The result is representative of three independent experiments. (C) The total RNA was analyzed by real-time PCR in triplicate for the expression of the IL-6 mRNA in wild-type RAW264.7 cells (WT-RAWs), co-cultured RAW264.7 cells (C-RAWs), wild-type 4T1 cells (WT-4T1s) and co-cultured 4T1 cells (C-4T1s). The results were normalized to the expression of the housekeeping gene β-actin. The data are expressed as fold changes in mRNA expression with respect to the wild-type cells.
As shown in Figure 2A, the crosstalk between RAW264.7 cells and 4T1 cells can indeed induce IL-6 secretion in the co-cultured cells; however, the source of IL-6 remains unknown. To determine the source of IL-6 produced under conditions of co-culture, IL-6 mRNA expression was detected by real-time PCR in WT-RAWs, C-RAWs, wild-type 4T1 cells (WT-4T1s) and C-4T1s. C-RAWs showed a 4420-fold increase of IL-6 mRNA expression in comparison with WT-RAWs (Figure 2C), whereas C-4T1s showed a 115-fold increase of IL-6 mRNA expression in comparison with WT-4T1s (Figure 2D). These data indicate that the crosstalk between RAW264.7 and 4T1 cells can promote the IL-6 secretion of both C-RAWs and C-4T1s; furthermore, C-RAWs are the main producers of IL-6. Together, these data suggest that the IL-6 produced by C-RAWs plays a major role in the induction of M2d macrophage generation in the co-culture.
Binding of Fra-1 to the IL-6 promoter regulates IL-6 expression in RAWs, thus affecting the generation of M2d macrophages
Many studies have reported that Fra-1 plays an important role in tumor progression and metastasis. To determine whether the transcription factor Fra-1 is involved in the generation of M2d macrophages, we detected the expression of Fra-1 in WT-RAWs and C-RAWs by real-time PCR and western blot analysis. The expression of both Fra-1 mRNA (right) and Fra-1 protein (left) increased in C-RAWs compared with that of WT-RAWs (Figure 3A).
Binding of Fra-1 to the IL-6 promoter regulates IL-6 expression in RAWs, thus affecting the generation of M2d macrophages. (A) Total RNA and cell extracts were prepared from C-RAWs and WT-RAWs. Expression levels of Fra-1 mRNA and protein in C-RAWs versus WT-RAWs (left) and equal amounts of cell extracts (5 μg) were subjected to western blot analysis with anti-Fra-1 and β-actin antibodies; the results are representative of three independent experiments. Right: total RNA was analyzed by real-time PCR in triplicate for the expression of Fra-1 mRNA. The data are expressed as fold changes in mRNA expression with respect to the wild-type cells. (B) Left: cell extracts from C-siFra-RAWs and C-sicon-RAWs were analyzed by western blot for the Fra-1 proteins; the results are representative of three independent experiments. Right: total RNA from siFra-RAWs and sicon-RAWs was analyzed by real-time PCR in triplicate for the expression of Fra-1 mRNA. The data are expressed as a percentage of the mRNA expression of sicon-RAWs. (C) Flow cytometric analysis of the percentage of M2d macrophages in the siFra-RAWs + 4T1s co-culture (1:4 RAWs:4T1s). The percentage of M2d macrophages in the sicon-RAWs + 4T1s co-culture is shown for comparison. The percentage of M2d macrophages was detected at different time points as indicated. The results are representative of three independent experiments. (D) Total RNA was prepared from C-siFra-RAWs and C-sicon-RAWs. RT-PCR analysis was carried out using the CD80-, CD86-, MHC-II-, IL-15- or β-actin-specific primers. The results are representative of three independent experiments. (E) IL-6 ELISA on the cell-free supernatant of siFra-RAWs + 4T1s co-culture or sicon-RAWs + 4T1s co-culture (1:4 RAWs:4T1s) at the indicated time points using sicon-RAWs + 4T1s co-culture as the control. The results are expressed in ng/ml. Columns are representative of three independent experiments carried out in quadruplicate. Bars, means ± SD. * P < 0.05 when compared with controls. (F) Total RNA from C-siFra-RAWs and C-sicon-RAWs was analyzed by real-time PCR in triplicate for the expression of Fra-1 mRNA. The data are expressed as a percentage of the mRNA expression of C-sicon-RAWs. (G) ChIP-enriched DNAs using anti-Fra-1 (line 3) or isotype IgG (line2) antibody were prepared from C-RAWs, WT-RAWs, C-siFra-RAWs and C-sicon-RAWs. DNA fragments of IL-6 promoter (−534 to −197) were analyzed by PCR (top) and real-time PCR (bottom). In each case, the input DNA (1:100 dilutions) was used as a positive control (line 1). For real-time PCR, left: the data are expressed as fold changes in binding to the IL-6 promoter with respect to WT-RAWs; right: data are expressed as a percentage of binding to the IL-6 promoter of C-sicon-RAWs.
After demonstrating the overexpression of Fra-1 in C-RAWs, we proceeded to assess whether knockdown Fra-1 expression in RAW264.7 cells might interfere with the generation of M2d macrophages in co-cultured cells. We performed RNA interference in RAW264.7 cells by transfecting siRNA specific for mouse Fra-1 (referred to as siFra-RAWs) or a negative control siRNA (referred to as sicon-RAWs). The efficiency of RNA interference was confirmed by real-time PCR and western blot analyses (Figure 3B). The expression of Fra-1 mRNA in siFra-RAWs was 30% of sicon-RAWs (Figure 3B, right). Because WT-RAWs express little of the Fra-1 protein, we co-cultured siFra-RAWs or sicon-RAWs with 4T1 cells for 72 h. Subsequently, co-cultured siFra-RAWs (referred to as C-siFra-RAWs) or co-cultured sicon-RAWs (referred to as C-sicon-RAWs) were isolated from the co-cultured cells. RNA interference greatly reduced the expression of Fra-1 protein in C-siFra-RAWs compared to C-sicon-RAWs (Figure 3B, left). We next detected the percentage of M2d macrophages in the co-culture of siFra-RAWs and 4T1 cells, comparing it with that in the co-culture of sicon-RAWs and 4T1 cells at different time points. As shown in Figure 3C, knockdown of Fra-1 expression greatly reduced the percentage of M2d macrophages in the co-culture at all time points. The results were confirmed by analyzing the mRNA levels of the co-stimulatory molecules CD80 and CD86 and of MHC-II and IL-15 in both C-siFra-RAWs and C-sicon-RAWs. CD80, CD86, MHC-II and IL-15 mRNA levels were consistently higher in C-siFra-RAWs compared with those in C-sicon-RAWs (Figure 3D). These data show that knockdown of Fra-1 expression reduces the phenotypic and functional characteristics of M2d macrophages, thereby preventing the generation of M2d macrophages in the co-culture.
As described previously, the IL-6 produced by C-RAWs plays a major role in inducing M2d macrophage generation via an autocrine mechanism. The IL-6 promoter is composed of a variety of regulatory elements, and the binding site for AP-1 is essential for the induction of IL-6. To investigate whether Fra-1 may regulate the expression of IL-6 in C-RAWs and thus affect the generation of M2d macrophages, we analyzed IL-6 protein levels in culture supernatant obtained from the co-culture of siFra-RAWs and 4T1 cells at 24, 48, 72 and 96 h time points. As shown in Figure 3E, knockdown of Fra-1 expression reduced the levels of IL-6 in the co-culture of siFra-RAWs and 4T1 cells compared with the control at all time points. The results were confirmed by analyzing the IL-6 mRNA levels in C-siFra-RAWs and C-sicon-RAWs. The level of IL-6 mRNA in C-siFra-RAWs was 67.8% of that in C-sicon-RAWs (Figure 3F). These data suggest that knockdown of Fra-1 downregulates IL-6 gene expression and protein secretion in C-siFra-RAWs.
We have demonstrated that C-RAWs express high levels of IL-6 mRNA and protein, and that knockdown of Fra-1 downregulates IL-6 gene expression and IL-6 protein secretion in C-siFra-RAWs. To clarify the molecular mechanisms of IL-6 gene expression in C-RAWs, we further investigated whether IL-6 expression in C-RAWs is regulated by the enhanced binding of Fra-1 to the IL-6 promoter. We therefore performed ChIP assays in C-RAWs, WT-RAWs, C-siFra-RAWs and C-sicon-RAWs. Immunoprecipitations of the cross-linked protein-DNA complexes with an antibody against Fra-1 were followed by analysis of the immunoprecipitated DNA by PCR and real-time PCR using IL-6 promoter-specific primers spanning from −534 to −197 surrounding the AP-1 site of mouse IL-6 promoter. As shown in Figure 3G, ChIP assays indicated that the binding of Fra-1 to IL-6 promoter was much greater in C-RAWs compared with that in the matched WT-RAWs (left), which correlated with the increased expression of IL-6 in C-RAWs (Figure 2A and 2C). The binding of Fra-1 to the IL-6 promoter was much lower in C-siFra-RAWs compared with that in the matched C-sicon-RAWs (Figure 3G, right), which correlated with the downregulation of IL-6 expression in C-siFra-RAWs (Figure 3E and 3F). These results support that Fra-1 binding to the IL-6 promoter regulates IL-6 expression in RAWs, thereby affecting the generation of M2d macrophages.
Discussion
Fra-1 is thought to play an important role in tumorigenesis and progression, and it is important for the motility and invasion of mesothelioma 33 and mammary 32, lung 34, 35, colon 36 and brain 37 cancer cells. However, the functional role of Fra-1 in the generation of M2d macrophages was unknown. Our study investigated the role of Fra-1 in this process, and the results revealed that the binding of Fra-1 to the IL-6 promoter regulated IL-6 expression in RAWs, thus affecting the generation of M2d macrophages. Tumor development is a consequence of the provision of a fertile environment (the soil) in which compatible tumor cells (the seed) can proliferate. Therefore, the TME plays an important role in allowing the tumor to express its full neoplastic phenotype. Mantovani et al. 12 reported that M2d macrophages represent the major inflammatory component of the infiltrate and considered them an important part of the inflammatory circuits that promote tumor progression. M2d macrophages are recruited at the tumor site by tumor-derived chemotactic factors such as CCL2. A series of studies have reported that M2d macrophages can promote tumor progression and metastasis 1, 5, 8, 38; however, the exact mechanism of macrophage polarization into M2d macrophages has not been demonstrated. It is proposed that the TME induces macrophages to adopt a tropic role that facilitates tumor metastasis 1; therefore, we hypothesized that tumor cell lines may skew macrophage differentiation into M2d macrophages in vitro. To test the hypothesis, we performed co-culture experiments and inducing experiments with RAW264.7 cells and 4T1 cells. The results revealed that 4T1 cells could skew RAW264.7 cell differentiation into M2d macrophages, which expressed low levels of mRNA for the co-stimulatory molecules CD80 and CD86, MHC-II, IL-15, IL-12p35, TNF-α and iNOS. The proteins CD80 and CD86 are molecules expressed on antigen-presenting cells that provide co-stimulatory signals necessary for T-cell activation and survival. The MHC class II presents antigens to T cells to induce strong immune reactions. IL-12p35, TNF-α and IL-15 are proinflammatory cytokines secreted by M1 macrophages. Hence, our findings are consistent with the conclusion that M2d macrophages are immunosuppressive cells in the TME 1. Therefore, the crosstalk between RAW264.7 cells and 4T1 breast tumor cells is critical for the switch that maintains the promalignancy phenotype of M2d macrophages.
As described above, IL-6 is expressed in numerous cancers and is involved in tumor progression; high circulating IL-6 levels are a marker of poor prognosis in breast cancer and myeloma patients. Our findings revealed that co-cultured cells secreted drastically higher levels of IL-6 compared with pure cell populations at all time points, which was mainly attributed to RAW264.7 cells expressing extraordinarily high levels of IL-6 mRNA after co-culture with 4T1 cells. IL-6 has been reported to be involved in the tumor-mediated regulation of DC differentiation 39; in another study, IL-6 was found to suppress DC maturation in vivo 40. To determine the role of IL-6 in promoting M2d macrophage generation, we added a neutralizing anti-mouse IL-6 antibody in the co-culture of RAW264.7 cells and 4T1 cells. The results revealed that the blockage of IL-6 could inhibit the generation of M2d macrophages. Together, these results suggest that IL-6 plays a pivotal role in inducing the generation of M2d macrophages and that IL-6 is mainly produced by RAW264.7 cells in the co-culture. Several studies have reported that many tumor cells produce IL-6 39, 41, 42, which is consistent with our findings that 4T1 cells secrete a high level of IL-6. Cancer cells that are exposed to IL-6 or secrete the cytokine as an autocrine factor show enhanced malignant features, such as an enhanced capacity to invade the extracellular matrix and increased drug resistance 16, 43, 44. IL-6 binds to a heterodimeric receptor that contains the ligand-binding IL-6Rα chain and the common cytokine receptor signal-transducing subunit gp130 15. Accordingly, inactivation of the gp130 protein has been found to reduce the aggressiveness of breast cancer cells in vivo 45. Garcia-Tunon et al. 46 suggested not only that breast tumor cells produce more IL-6 than normal breast epithelial cells but also that the response of the tumor cells to this interleukin is greater. Together, the drastically high level of IL-6 secreted in the co-culture of RAW264.7 cells and 4T1 cells plays a pivotal role in inducing the generation of M2d macrophages and in enhancing the malignant features of tumor cells. This may lead to an even greater secretion of IL-6 in the co-culture. We propose that a 'feed forward' loop exists between RAW264.7 cells and 4T1 cells to promote enhanced malignant features of 4T1 cells. Blockage of the loop may represent an innovative anticancer strategy.
Deregulated IL-6 expression has been observed in M2d macrophages; nevertheless, the importance of IL-6 per se for M2d macrophage generation is not entirely clear, and it is possible that IL-6 is only one of several genes that act in concert to promote M2d macrophage generation and are regulated by the same upstream signals. Therefore, IL-6 may represent a surrogate cytokine for M2d macrophage generation, and interference with IL-6 alone as a therapeutic method may not work, because IL-6 is only one of several factors with a similar function. We therefore suggest that understanding the regulation of IL-6 gene expression in M2d macrophages may lead to elucidation of the upstream factors that deregulate the expression of a whole set of genes involved in M2d macrophage generation. These upstream factors should be far more desirable points of interference for novel therapies than IL-6 itself. As the first step to delineate the molecular mechanisms leading to deregulated IL-6 gene expression in M2d macrophages, we sought to define the transcription factors that constitutively activate the IL-6 gene in M2d macrophages. Fra-1 is thought to play an important role in tumorigenesis and progression. Belguise et al. 32 showed that Fra-1 is highly expressed in the more invasive estrogen receptor negative (ER−) breast cancer cell lines compared with ER+ cell lines. Similarly, our findings revealed that C-RAWs showed constitutive expression of Fra-1, which indicates a malignant feature of the C-RAWs. Our data also showed that knockdown of Fra-1 expression greatly inhibited the propensity of RAW264.7 cells to differentiate into M2d macrophages and reversed the immunosuppressive characteristics of M2 macrophages. These findings suggest that Fra-1 plays an important role in the generation of M2d macrophages. The data obtained from C-siFra-RAWs revealed that knockdown of Fra-1 downregulated IL-6 gene expression. This observation is in line with the conclusions that the IL-6 promoter is composed of a variety of overlapping regulatory elements and that the binding site for AP-1 is essential for the induction of IL-6. Thus, we propose that the binding of Fra-1 to the IL-6 promoter regulates IL-6 gene expression. The data obtained from ChIP assays confirmed this notion.
In summary, we identified Fra-1 as being constitutively active in M2d macrophages and demonstrated that Fra-1 binds to the IL-6 promoter region. Fra-1 binding to the IL-6 promoter led to increased IL-6 expression, thereby affecting the generation of M2d macrophages. Furthermore, our results strongly support the idea that blockage of IL-6 and its upstream factor Fra-1 can inhibit the generation of M2d macrophages. This appears to be part of the immunosuppressive mechanisms correlated with tumor progression. Our study provides new insight into how to reverse M2d macrophage-induced immune tolerance to improve the efficacy of immunotherapy in cancer.
Materials and Methods
Cell culture and cell sorting
RAW264.7 cells, 4T-1 cells and Mφs were incubated in IMEM (GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum (MDgenics, St Louis, MO, USA) and 100 U per ml of penicillin-streptomycin. For co-culture experiments, RAW264.7 cells were co-cultured with 4T1 cells at a 1:4 ratio in complete medium (CM) for 24 h, 48 h, 72 h or 96 h. In inducing experiments, RAW264.7 cells were incubated with the supernatant of 4T1 cells (diluted 1:5) for 72 h (added at hours 0, 24 and 48). In the neutralization experiment, co-cultured cells were maintained in CM with rabbit anti-mouse IL-6 (500 ng/ml) or isotype IgG antibody (500 ng/ml) (added at hours 0, 24 and 48). In some experiments, WT-RAWs and 60-h co-cultured cells were treated with or without LPS (100 ng/ml) overnight. For single-cell analysis, a minimum of 1 × 107 total C-RAWs and C-4T1s (at 72-h time point) were stained using monoclonal antibodies. Phycoerythrin (PE)-conjugated anti-mouse F4/80 (eBioscience, San Diego, CA, USA), RAW264.7 cells (F4/80+ cells) and 4T1 cells (F4/80− cells) were sorted by FACS Aria (BD Biosciences, San Jose, CA, USA), with a routine purity of 94.3% and 99.7%, respectively (Figure 1C). Mφs were obtained from female BALB/c mice at 6–8 weeks of age. The RAW 264.7 macrophage cell line was purchased from the School of Basic Medicine, Peking Union Medical College (Beijing, China); the murine 4T1 breast carcinoma cells were kindly provided by Dr Ostrand-Rosenberg S (University of Maryland, College Park, Maryland, USA); LPS was from Sigma (St, Louis, MO, USA); rabbit anti-mouse IL-6 was from eBioscience; rabbit unspecific IgG (isotype IgG antibody) was from Newprobe biotechnology (Beijing, China).
ELISA
The determination of murine IL-6 in the cell supernatants was carried out using ELISA kits purchased from R&D systems Inc (Minneapolis, MN, USA).
Flow cytometry
M2d macrophages were stained using monoclonal antibodies, PE-conjugated anti-mouse F4/80 (eBioscience) and FITC-conjugated anti-mouse CD206 (Biolegend, San Diego, CA, USA), according to the manufacturers' instructions. A total of 10 000 events were analyzed using BD CellQuest software. All samples were processed using the BD FACS Calibur flowcytometer.
RT-PCR analysis
Total RNA was extracted from cultured cells using the RNA extracting reagent Trizol (Dingguo Biotechnology, Beijing). The reverse transcription reaction from 1 μg of RNA template was carried out using reverse transcription reagents (Tiangen Biotech, Beijing, China) as per the manufacturer's instructions. PCR amplifications were carried out as follows: 2 min at 95 °C; 35 cycles of 30 s at 95 °C, 30 s at annealing temperature and 1 min at 72 °C; followed by 5 min at 72 °C. Primers (Invitrogen, Shanghai, China) used in the PCR experiments were as follows: CD80, annealing temperature 53 °C, amplicon length 650 bp, forward 5′-TGG TGC TGT CTG TCA TTG-3′, reverse 5′-GGT AAG GCT GTT GTT TGT T-3′; CD86, annealing temperature 53 °C, amplicon length 435 bp, forward 5′-CAG TCA GGA TGG GAG TGG TA-3′, reverse 5′-TTG AGT ACT TGG CTG TCT TA-3′; H-2, annealing temperature 57 °C, amplicon length 544 bp, forward 5′-ATC TAA TCA GGG CTA CCA CG-3′, reverse 5′-GAC TCT AAA CGG CTC TTC G-3′; IL-15, annealing temperature 52 °C, amplicon length 313 bp, forward 5′-GTG TTT GGA AGG CTG AGT T-3′, reverse 5′-CAC AAG TAG CAC GAG ATG G-3′; and β-actin, annealing temperature 54 °C, amplicon length 281 bp, forward 5′-CGT TGA CAT CCG TAA AGA CC-3′, reverse 5′- AAC AGT CCG CCT AGA AGC AC-3′. The PCR experiments were carried out with GoTaq Colorless Master Mix (Promega) as per the manufacturer's instructions.
Real-time PCR
Total RNA and cDNA were generated as described in the RT-PCR analysis, and quantitative PCR was performed using the QuantiFast SYBR Green PCR kit (Qiagen, Valencia, CA, USA) and detected by the DA7600 Real-time Nucleic Acid Amplification Fluorescence Detection System (Da An Gene, Guangdong, China). Primers (Takara, Dalian, China) used in the real-time PCR were Fra-1, forward 5′-CCA GGG CAT GTA CCG AGA CTA-3′, reverse 5′-GAT GCT TGG CAC AAG GTG GA-3′; IL-6, forward 5′-CCA CTT CAC AAG TCG GAG GCT TA-3′, reverse 5′-GCA AGT GCA TCA TCG TTG TTC ATA C-3′; IL-10, forward 5′-GGG CCA GTA CAG CCG GGA AG-3′, reverse 5′-CTG GCT GAA GGC AGT CCG CA-3′; IL-12p35, forward 5′-GCA CCC GCG TCG TGA CCA TC-3′, reverse 5′-GCC CAC CAG GCC AAG ACC AC-3′; TNF-α, forward 5′-AAG GCC GGG GTG TCC TGG AG-3′, reverse 5′-AGG CCA GGT GGG GAC AGC TC-3′; TGF-β, forward 5′-TGG TGG ACC GCA ACA ACG CC-3′, reverse 5′-GGG GGT TCG GGC ACT GCT TC-3′; CCL2, forward 5′-GAG GAA GGC CAG CCC AGC AC-3′, reverse 5′-TGG ATG CTC CAG CCG GCA AC-3′; CCL22, forward 5′-GTG CCG ATC CCA GGC AGG TC-3′, reverse 5′-GGC GTC GTT GGC AAG GCT CT-3′; iNOS, forward 5′-CCG CTG CCT TCC TGC TGT CG-3′, reverse 5′-CCT CCG AGG GGG TGT GGT CC-3′; and Arg1, forward 5′-AGA GAC CAC GGG GAC CTG GC-3′, reverse 5′-TGG ACC TCT GCC ACC ACA CC-3′. The relative expression levels of the target genes (Fra-1, IL-6, etc.) against that of the β-actin were defined as −ΔCt =−(CtTarget−Ctβ-actin). The target mRNA/β-actin mRNA ratio was calculated as 2−ΔCtaccording to the manufacturer's specifications. All real-time results were expressed as fold changes in mRNA expression with respect to the control cells. All results were normalized to the expression of the housekeeping gene β-actin in the PCR reactions. Data are from two independent experiments carried out in triplicate.
Chromatin immunoprecipitation (ChIP) assay
A ChIP assay was performed using the EZ-ChIP kit (Millipore, Temecula, CA, USA). Antibodies used for immunoprecipitation were rabbit anti-mouse Fra-1(N-17)X (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or isotype IgG antibody (Newprobe Biotechnology) as a negative control. Primers for the amplification of a Fra-1-binding site-specific, proximal region (nucleotides −296 to −290) within the murine were 5′- GAA AAA ACT CAG GTC AGA AC-3′ and 5′- AAG AAT CAC AAC TAG GAA GG-3′, with an expected size of 338 bp. Real-time PCR was performed as described previously. PCR amplification was carried out as follows: 1 cycle at 95 °C for 2 min followed by 35 cycles at 95 °C for 30 s and at an annealing temperature of 60 °C for 10 s and 72 °C for 22 s, and then subjected to a final elongation at 72 °C for 5 min.
Western blot analysis
Cell extracts were prepared by nuclear-cytosol extraction kit (Keygen, Nanjing, China) and measured using a BCA protein assay kit (Keygen). Lysates were separated by SDS/PAGE (10% acrylamide). Immunoblotting was carried out with rabbit anti-mouse Fra-1 (N-17) X (Santa Cruz Biotechnology) or rabbit anti-mouse β-actin (Biosynthesis Biotechnology, Beijing, China). After being washed, HRP-conjugated anti-rabbit IgG secondary antibodies (Promega, Madison, WI, USA) were incubated with the membranes, washed and detected with Immobilon Western Chemiluminescent HRP substrate (Millipore, Bedford, MA, USA).
RNA interference
To silence the Fra-1 expression, RAW264.7 cells were transfected with siRNA specific for mice Fra-1 (RiboBio Co, Guangzhou, China) or the negative control siRNA using HiperFect transfection reagent (Qiagen) according to the manufacturer's specifications. After 48 and 72 h of incubation, cells were collected for RNA analysis and western blot, respectively.
Statistics
Data are shown as the means±SD. To compare IL-6 content among multiple test groups, we performed a one-way ANOVA followed by Newman–Keuls test. We used an unpaired t-test to calculate two-tailed P-values to estimate the statistical significance of differences between two groups. All of the tests were implemented in SPSS 11.5 (SPSS). P-values less than 0.05 were considered significant.
References
Pollard JW . Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4:71–78.
Balkwill F, Mantovani A . Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420:860–867.
Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L . The origin and function of tumor-associated macrophages. Immunol Today 1992; 13:265–270.
Sica A, Schioppa T, Mantovani A, Allavena P . Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006; 42:717–727.
Zhang B, Wang J, Gao J, et al. Alternatively activated RAW264.7 macrophages enhance tumor lymphangiogenesis in mouse lung adenocarcinoma. J Cell Biochem 2009; 107:134–143.
Martinez FO, Sica A, Mantovani A, Locati M . Macrophage activation and polarization. Front Biosci 2008; 13:453–461.
Condeelis J, Pollard JW . Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124:263–266.
Bingle L, Brown NJ, Lewis CE . The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002; 196:254–265.
Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 2006; 116:2132–2141.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M . The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25:677–686.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A . Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549–555.
Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007; 110:4319–4330.
Mantovani A, Allavena P, Sica A . Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer 2004; 40:1660–1667.
Kishimoto T . Interleukin-6: from basic science to medicine -- 40 years in immunology. Annu Rev Immunol 2005; 23:1–21.
Conze D, Weiss L, Regen PS, et al. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res 2001; 61:8851–8858.
Salem M, Elbaz O, Zahran M, et al. Malignancy: identification of predictors of disease status and progression in patients with myeloma (MM). Hematology 2000; 5:41–45.
Thavasu PW, Ganjoo RK, Maidment SA, et al. Multiple myeloma: an immunoclinical study of disease and response to treatment. Hematol Oncol 1995; 13:69–82.
Knupfer H, Preiss R . Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat 2007; 102:129–135.
Foti E, Ferrandina G, Martucci R, et al. IL-6, M-CSF and IAP cytokines in ovarian cancer: simultaneous assessment of serum levels. Oncology 1999; 57:211–215.
Allavena P, Piemonti L, Longoni D, et al. IL-10 prevents the generation of dendritic cells from CD14+ blood monocytes, promotes the differentiation to mature macrophages and stimulates endocytosis of FITC-dextran. Adv Exp Med Biol 1997; 417:323–327.
Oyama T, Ran S, Ishida T, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 1998; 160:1224–1232.
Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP . Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 1999; 5:2963–2970.
Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92:4150–4166.
Dendorfer U, Oettgen P, Libermann TA . Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol 1994; 14:4443–4454.
Libermann TA, Baltimore D . Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 1990; 10:2327–2334.
Zerbini LF, Wang Y, Cho JY, Libermann TA . Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res 2003; 63:2206–2215.
Chinenov Y, Kerppola TK . Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 2001; 20:2438–2452.
Milde-Langosch K . The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 2005; 41:2449–2461.
Wisdom R, Verma IM . Proto-oncogene FosB: the amino terminus encodes a regulatory function required for transformation. Mol Cell Biol 1993; 13:2635–2643.
Shaulian E, Karin M . AP-1 as a regulator of cell life and death. Nat Cell Biol 2002; 4:E131–E136.
Belguise K, Kersual N, Galtier F, Chalbos D . FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 2005; 24:1434–1444.
Ramos-Nino ME, Blumen SR, Pass H, Mossman BT . Fra-1 governs cell migration via modulation of CD44 expression in human mesotheliomas. Mol Cancer 2007; 6:81.
Adiseshaiah P, Vaz M, Machireddy N, Kalvakolanu DV, Reddy SP . A Fra-1-dependent, matrix metalloproteinase driven EGFR activation promotes human lung epithelial cell motility and invasion. J Cell Physiol 2008; 216:405–412.
Adiseshaiah P, Lindner DJ, Kalvakolanu DV, Reddy SP . FRA-1 proto-oncogene induces lung epithelial cell invasion and anchorage-independent growth, but is insufficient to promote tumor growth in vivo. Cancer Res 2007; 67:6204–6211.
Andreolas C, Kalogeropoulou M, Voulgari A, Pintzas A . Fra-1 regulates vimentin during Ha-RAS-induced epithelial mesenchymal transition in human colon carcinoma cells. Int J Cancer 2008; 122:1745–1756.
Debinski W, Gibo DM . Fos-related antigen 1 modulates malignant features of glioma cells. Mol Cancer Res 2005; 3:237–249.
DeNardo DG, Barreto JB, Andreu P, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91–102.
Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34+ progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood 1998; 92:4778–4791.
Park SJ, Nakagawa T, Kitamura H, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 2004; 173:3844–3854.
Nabarro S, Himoudi N, Papanastasiou A, et al. Coordinated oncogenic transformation and inhibition of host immune responses by the PAX3-FKHR fusion oncoprotein. J Exp Med 2005; 202:1399–1410.
Yu H, Kortylewski M, Pardoll D . Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7:41–51.
Asgeirsson KS, Olafsdottir K, Jonasson JG, Ogmundsdottir HM . The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine 1998; 10:720–728.
Sehgal PB, Tamm I . Interleukin-6 enhances motility of breast carcinoma cells. EXS 1991; 59:178–193.
Selander KS, Li L, Watson L, et al. Inhibition of gp130 signaling in breast cancer blocks constitutive activation of Stat3 and inhibits in vivo malignancy. Cancer Res 2004; 64:6924–6933.
Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M . IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology 2005; 47:82–89.
Acknowledgements
We would like to thank Jie Zhang, Changlin Li, Ying Xing and Liming Sun (Nankai University, China) for technical assistance and Zhen Liu for linguistic support. This work was supported by the National Basic Research Program of China (973 Program, 2007CB914804), the National High Technology Research and Development Program of China (863 Program, 2007AA021010), the Foundation of the Ministry of Education of China for Returned Scholars, the Key Project of the Science & Technology Pillar Program of Tianjin (09JCYBJC10800) and the Innovative Research Foundation of Nankai University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Ni, H., Lan, L. et al. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res 20, 701–712 (2010). https://doi.org/10.1038/cr.2010.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2010.52
Keywords
This article is cited by
-
Role of myeloid cells in ischemic retinopathies: recent advances and unanswered questions
Journal of Neuroinflammation (2024)
-
Label-free macrophage phenotype classification using machine learning methods
Scientific Reports (2023)
-
Macrophage subsets and their role: co-relation with colony-stimulating factor-1 receptor and clinical relevance
Immunologic Research (2023)
-
A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression
Journal of Experimental & Clinical Cancer Research (2022)
-
The role of tumor-associated macrophages in osteosarcoma progression – therapeutic implications
Cellular Oncology (2021)