Abstract
Background/objective:
Blood proportions of fatty acids (FAs) and FA-ratios reflecting desaturase activity are associated with the risk of chronic diseases like type 2 diabetes mellitus or cardiovascular diseases. Biomarkers of dyslipidemia are considered as potential mediators of this association. We evaluated associations of erythrocyte membrane proportions of individual disease-related polyunsaturated fatty acids (PUFAs), trans-FAs, dairy-derived saturated FAs (SFAs) (15:0, 17:0) and FA-ratios with biomarkers of dyslipidemia (high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, non-HDL-cholesterol, triglycerides).
Subjects/methods:
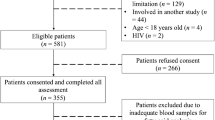
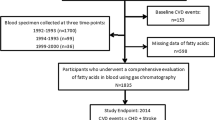
We conducted a cross-sectional analysis of a subsample (n=1759) of the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Associations of individual FAs and FA-ratios with plasma biomarkers of dyslipidemia were evaluated by linear multivariable regression.
Results:
Most notably, FA-ratios reflecting activity of Δ6-desaturase (D6D) and stearoyl-coenzyme A-desaturase (SCD) were positively associated with triglyceride and LDL-cholesterol concentrations (adjusted means (95% confidence interval (CI)) of triglycerides (mg/dl) across D6D tertiles: men—102 (94.7–110), 111 (104–120), 144 (134–156) and women—73.5 (70.0–77.2), 82.9 (79.0–86.9), 94.2 (89.7–98.9)); across SCD tertiles: men—99.0 (91.8–107), 115 (107–124), 144 (134–156) and women—72.4 (69.0–76.0), 81.5 (77.8–85.5), 97.2 (92.6–102)), whereas inverse associations with triglycerides were observed for the estimated Δ5-desaturase (D5D) activity (adjusted means (95% CI) of triglycerides (mg/dl) across D5D tertiles: men—128 (119–138), 121 (113–131), 106 (97.9–114) and women—92.0 (87.6–96.6), 82.8 (78.9–86.9), 75.3 (71.6–79.1), P-values for trend at least 0.0006). Furthermore, we observed generally weaker and less consistent associations of dairy-derived SFAs (mainly 17:0) with triglycerides and HDL-cholesterol. Individual PUFAs and trans-FAs were, if at all, only weakly associated with dyslipidemia markers.
Conclusions:
Our findings suggest that triglyceride and LDL-cholesterol concentrations may be mediators that link intake and metabolism of FAs to metabolic risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Riserus U, Willett WC, Hu FB . Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 2009; 48: 44–51.
Erkkila A, de Mello VD, Riserus U, Laaksonen DE . Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res 2008; 47: 172–187.
Hodson L, Skeaff CM, Fielding BA . Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47: 348–380.
Bordoni A, Di Nunzio M, Danesi F, Biagi PL . Polyunsaturated fatty acids: from diet to binding to ppars and other nuclear receptors. Genes Nutr 2006; 1: 95–106.
Fernandez ML, West KL . Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 2005; 135: 2075–2078.
Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F . Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr 2012; 107 ((Suppl 2)): S201–S213.
Jacobson TA, Glickstein SB, Rowe JD, Soni PN . Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol 2012; 6: 5–18.
Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007; 86: 189–197.
Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B et al. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2008; 18: 503–510.
Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010; 92: 1214–1222.
Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EHJM, Doring F et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2011; 93: 127–142.
Mensink RP, Zock PL, Kester AD, Katan MB . Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003; 77: 1146–1155.
Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006; 91: 439–446.
Motoyama KR, Curb JD, Kadowaki T, El-Saed A, Abbott RD, Okamura T et al. Association of serum n-6 and n-3 polyunsaturated fatty acids with lipids in 3 populations of middle-aged men. Am J Clin Nutr 2009; 90: 49–55.
Kroger J, Schulze MB . Recent insights into the relation of Delta5 desaturase and Delta6 desaturase activity to the development of type 2 diabetes. Curr Opin Lipidol 2012; 23: 4–10.
Bjermo H, Riserus U . Role of hepatic desaturases in obesity-related metabolic disorders. Curr Opin Clin Nutr Metab Care 2010; 13: 703–708.
Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009; 41: 56–65.
Warensjo E, Jansson JH, Berglund L, Boman K, Ahren B, Weinehall L et al. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case–control study. Br J Nutr 2004; 91: 635–642.
Smedman AEM, Gustafsson IB, Berglund LGT, Vessby BOH . Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr 1999; 69: 22–29.
Mozaffarian D, Aro A, Willett WC . Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 2009; 63 ((Suppl 2)): S5–S21.
Mozaffarian D, Clarke R . Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009; 63: S22–S33.
Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC . Trans fatty acids and cardiovascular disease. N Engl J Med 2006; 354: 1601–1613.
Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010; 153: 790–799.
Yu DX, Sun Q, Ye XW, Pan A, Zong G, Zhou YH et al. Erythrocyte trans-fatty acids, type 2 diabetes and cardiovascular risk factors in middle-aged and older Chinese individuals. Diabetologia 2012; 55: 2954–2962.
Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY et al. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013; 97: 854–861.
Boeing H, Wahrendorf J, Becker N . EPIC-Germany—a source for studies into diet and risk of chronic diseases. Ann Nutr Metab 1999; 43: 195–204.
van Dieren S, Nothlings U, van der Schouw YT, Spijkerman AM, Rutten GE, van der AD et al. Non-fasting lipids and risk of cardiovascular disease in patients with diabetes mellitus. Diabetologia 2011; 54: 73–77.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Chow LS, Li S, Eberly LE, Seaquist ER, Eckfeldt JH, Hoogeveen RC et al. Estimated plasma stearoyl co-A desaturase-1 activity and risk of incident diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Metabolism 2013; 62: 100–108.
Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR . Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care 2008; 31: 982–988.
Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007; 115: 450–458.
Lindi V, Schwab U, Louheranta A, Laakso M, Vessby B, Hermansen K et al. Impact of the Pro12Ala polymorphism of the PPAR-gamma2 gene on serum triacylglycerol response to n-3 fatty acid supplementation. Mol Genet Metab 2003; 79: 52–60.
Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr 2012; 96: 1137–1149.
Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, Riboli E, Toniolo P . Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr 2000; 54: 367–372.
Hall WL . Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev 2009; 22: 18–38.
Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, Jakobsen MU et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 2011; 93: 684–688.
Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M . Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr 2009; 139: 1–4.
Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ . Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 2008; 197: 12–24.
Warensjo E, Jansson JH, Cederholm T, Boman K, Eliasson M, Hallmans G et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case–control study. Am J Clin Nutr 2010; 92: 194–202.
Sun Q, Ma J, Campos H, Hankinson SE, Manson JE, Stampfer MJ et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation 2007; 115: 1858–1865.
Kabagambe EK, Tsai MY, Hopkins PN, Ordovas JM, Peacock JM, Borecki IB et al. Erythrocyte fatty acid composition and the metabolic syndrome: a National Heart, Lung, and Blood Institute GOLDN study. Clin Chem 2008; 54: 154–162.
Counil E, Julien P, Lamarche B, Chateau-Degat ML, Ferland A, Dewailly E . Association between trans-fatty acids in erythrocytes and pro-atherogenic lipid profiles among Canadian Inuit of Nunavik: possible influences of sex and age. Br J Nutr 2009; 102: 766–776.
Kabagambe EK, Ordovas JM, Hopkins PN, Tsai MY, Arnett DK . The relation between erythrocyte trans fat and triglyceride, VLDL- and HDL-cholesterol concentrations depends on polyunsaturated fat. PLoS One 2012; 7: 15.
Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation 2006; 114: 209–215.
Thiebaut AC, Rotival M, Gauthier E, Lenoir GM, Boutron-Ruault MC, Joulin V et al. Correlation between serum phospholipid fatty acids and dietary intakes assessed a few years earlier. Nutr Cancer 2009; 61: 500–509.
Mendis S, Cruz-Hernandez C, Ratnayake WMN . Fatty acid profile of Canadian dairy products with special attention to the <i>trans</i>-octadecenoic acid and conjugated linoleic acid isomers. J AOAC Int 2008; 91: 811–819.
Langsted A, Freiberg JJ, Nordestgaard BG . Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008; 118: 2047–2056.
Warnick GR, Knopp RH, Fitzpatrick V, Branson L . Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem 1990; 36: 15–19.
Gillingham LG, Harding SV, Rideout TC, Yurkova N, Cunnane SC, Eck PK et al. Dietary oils and FADS1–FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr 2013; 97: 195–207.
Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Konigsrainer A et al. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 2009; 55: 2113–2120.
Sjogren P, Sierra-Johnson J, Gertow K, Rosell M, Vessby B, de Faire U et al. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008; 51: 328–335.
Zietemann V, Kroger J, Enzenbach C, Jansen E, Fritsche A, Weikert C et al. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Br J Nutr 2010; 104: 1748–1759.
Acknowledgements
We thank all EPIC participants for their contribution to the study and Ellen Kohlsdorf for data management. We are indebted to Hans Cremers for the excellent performance of the FA analysis. The recruitment phase of the EPIC-Potsdam Study was supported by the Federal Ministry of Science, Germany (01EA 9401) and the European Union (SOC 95201408 05F02). The present study was also supported by a grant from the German Research Foundation (DFG, SCHU 1516/5-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Rights and permissions
About this article
Cite this article
Jacobs, S., Schiller, K., Jansen, E. et al. Association between erythrocyte membrane fatty acids and biomarkers of dyslipidemia in the EPIC-Potsdam study. Eur J Clin Nutr 68, 517–525 (2014). https://doi.org/10.1038/ejcn.2014.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.18
This article is cited by
-
Estimated Elovl6 and delta-5 desaturase activities might represent potential markers for insulin resistance in Japanese adults
Journal of Diabetes & Metabolic Disorders (2022)
-
Association of desaturase activity and C-reactive protein in European children
Pediatric Research (2017)
-
Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair
Scientific Reports (2017)
-
Trans-fatty acid levels in erythrocytes in Europe
European Journal of Nutrition (2017)
-
Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study
BMC Medicine (2017)