Abstract
Carriers of reciprocal translocations (rcp) are known to be at risk for reproductive difficulties. Preimplantation genetic diagnosis (PGD) is one of the options these carriers have to try in order to fulfil their desire to have a child. In the present study, we retrospectively looked at the results of 11 years (1997–2007) of PGD for rcp in our center to improve the reproductive counseling of these carriers. During this period 312 cycles were performed for 69 male and 73 female carriers. The mean female age was 32.8 years, the mean male age 35.8 years. Most carriers were diagnosed with a translocation because of fertility problems or recurrent miscarriages, and most of them opted for PGD to avoid these problems. In 150 of the 312 cycles, embryo transfer (ET) was feasible and 40 women had a successful singleton or twin pregnancy. This gives a live birth delivery rate of 12.8% per started cycle and of 26.7% per cycle with ET. Owing to the large number of abnormal embryos, PGD cycles for rcp often lead to cancellation of ET, explaining the low success rate when expressed per cycle with oocyte pick-up. Once ET was feasible, the live birth delivery rate was similar to that of PGD in general at our center. PGD is therefore an established option for specific reciprocal translocation carriers.
Similar content being viewed by others
Introduction
Reciprocal translocations (rcp) are structural chromosome rearrangements affecting two or more chromosomes and are typically a balanced exchange of terminal segments between non-homologous chromosomes with no discernable gain or loss of material. With the exception of the relatively common t(11;22)(q23.2; q11.2),1, 2 any point of any chromosome may be involved which results in most translocations being unique and family-specific. The prevalence of individuals with a balanced rcp in unbiased newborn surveys is 0.17%;3, 4 however, approximately 3% of couples with a history of subfertility have a rcp.5, 6
A balanced rcp carrier typically has a normal phenotype; however, the production of gametes with chromosome imbalance following meiotic segregation of the rcp can be associated with an increased risk of recurrent spontaneous abortion, infertility, and offspring with mental and/ or physical disability.7 It is therefore generally accepted that rcp carriers should be referred to a genetic counselor, and in this context prenatal as well as preimplantation genetic diagnosis (PGD) might be discussed as appropriate options for couples who wish to try and have a healthy child using their own gametes.8
In PGD, single cells from embryos created in vitro are analyzed for a specific genetic defect and the aim is to obtain a pregnancy of an embryo not affected by a well-defined monogenic or chromosomal condition. Diagnosis can be made on first or second polar bodies from oocytes (when the woman is carrier) or on blastomeres from cleavage-stage embryos or on trophectoderm cells of a blastocyst. In case of rcp, the diagnosis can be made by fluorescence in situ-hybridization (FISH) or, more recently, by PCR-based techniques. As the blastomeres are in the interphase stage, the applied FISH technique does not allow the distinguishing of cells with the normal chromosomes from cells with the balanced complement.
In the present study, results of 11 years PGD–FISH on blastomeres for rcp are reported and these data will be helpful in the counseling of the carrier couples.
Materials and methods
Data from all requests and PGD cycles for rcp between January 1997 and December 2007 were analyzed.
Data on parental age, country of residence, reproductive history (number of previous pregnancies, miscarriages, live births, previous use of assisted reproductive techniques, duration of child wish) ascertainment of the diagnosis of the translocation, age at diagnosis, familial history, karyotype of the non-carrier partner and reason for opting for PGD were recorded for all couples who had at least one PGD cycle. For couples with a successful PGD cycle, the time between finishing the test work-up and delivery of the first child was also registered. For the couples who requested PGD but did not go through with it, parental age, country of residence, reproductive history and reason for inquiring about PGD was registered together with the reason why they finally did not have PGD.
For the PGD cycles the following items were recorded: the number of cumulus oocyte complexes at oocyte pick-up (OPU), MII oocytes, 2 pro-nucleate (2PN) zygotes, successfully biopsied embryos, results of FISH analysis of the biopsied blastomeres, ratio of chromosomally normal embryos, number of transferred and cryopreserved embryos, positive hCG, ongoing pregnancy, live-birth delivery and outcome of the children.
All couples who started PGD were first seen by a clinical geneticist, a reproductive medicine specialist and a reproductive medicine counselor, and gave their written informed consent for the treatment as well as for the follow-up of the pregnancy and the children.
In order to detect all the unbalanced products of a rcp at least three FISH probes are required. Preceding clinical PGD, each couple's test was worked up using their own peripheral blood lymphocytes to assess the efficiency and specificity of the FISH probes selected for the rcp according to current recommended best practice.9 From 2004 onward, where it was possible to incorporate all the rcp probes in a single hybridization reaction, aneuploidy screening for chromosomes 13, 18, 21, X and Y (or subset not involved in the rcp) was included in a second hybridization reaction. We did not screen for other chromosomes as this would need a third hybridization reaction, which is more time consuming and less efficient with respect to the FISH procedure.
PGD requires an In vitro fertilization treatment with controlled ovarian stimulation, transvaginal OPU and Intracytoplasmic Sperm Injection (ICSI).10, 11, 12 On day 3, embryos with at least five cells are biopsied and preferentially two blastomeres are aspirated through a laser-drilled hole in the zona pellucida.13 The blastomeres are fixed on a slide using the HCl/Tween 20 method14 and FISH is performed as described by Staessen et al.15
Only embryos with a concordant normal/balanced result in the blastomeres were considered for embryo transfer (ET). In the majority of the cycles with ET, one or two embryos were transferred, but exceptionally three or four embryos were transferred. Post-ET surveillance consisted of a blood sample for hCG measurement at day 12 and pelvic ultrasound at 7 weeks (in case of positive hCG). All pregnant couples were counseled about the possibility of prenatal diagnosis with respect to the small risk of misdiagnosis (<1%) 16 and in some cases a maternal age risk for aneuploidy (especially when no aneuploidy screening was performed during PGD).
Data on pregnancy, delivery and children were acquired through questionnaires for the families and their doctors (obstetrician, pediatrician), and/or through follow-up visits at our center, as described previously.17
Statistical analyses
Data were analyzed according to the sex of the carrier and for the total group.
Categorical data are represented as number of events/cases and percentages for each study group. Comparisons of percentages among female and male carriers are presented as odds ratios (OR) with corresponding 95% confidence intervals (95% CI) for each comparison. Continuous data on age, birth parameters and gestational age are represented as mean and SD.
Results
Patients
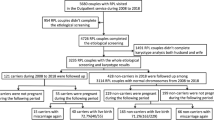
In a period of 11 years, 277 couples with 272 different translocations contacted the Center for Medical Genetics with a request for PGD for a rcp. The majority of these couples did not reside in Belgium at the time of their request (38.3% in Belgium, 57.0% in another European country and 4.7% outside of Europe).
Of the 277 couples, 135 did not proceed with PGD and 142 had at least one PGD cycle. Both groups had similar characteristics with respect to female and male age, and reasons why they considered PGD (summary in Table 1). Most couples considered PGD because of its possibility to overcome infertility and repeated miscarriages.
The reasons why some couples did not pursue PGD were various. In 23% (31/135) of the cases a PGD test was not possible; 11.9% (16/135) became spontaneously pregnant and therefore did not need PGD; 11.1% (15/135) had assisted conception without PGD and 7.4% (10/135) because of financial, personal or relationship problems. Of the remaining 46.7% (63/135) reasons included maternal age, adoption of a child, azoospermia and ovarian insufficiency.
The 69 male carrier couples who underwent PGD had a previous reproductive history of 84 pregnancies leading to 79 miscarriages and the birth of four healthy children and one child with multiple congenital anomalies due to an unbalanced translocation. It is noteworthy that of the four healthy children, only one was conceived spontaneously, whereas two others were children after ICSI treatment and one was conceived with the sperm of a donor. During one of the two ICSI pregnancies, prenatal diagnosis was performed showing a balanced translocation.
At the moment of their first appointment at the Center for Medical Genetics, the men and their spouses had a median period of trying for a child of 3.0 years (range 0.5–10 years).
For the 73 female carriers we registered 176 previous pregnancies, ending in 160 miscarriages and the birth of 17 children (15 singletons and 1 twin). Of these 17 children, 5 carried an unbalanced translocation and presented with multiple congenital anomalies leading to early lethality in 1. Two of the healthy children were conceived by ICSI. Eight of the healthy children were prenatally karyotyped showing 5 normal and 3 balanced karyotypes.
The couples’ median time of trying for a child was 2.0 years (range 0-10 years) when they first visited our Center.
PGD treatment cycles
The data on the PGD cycles are summarized in Table 2. Results according to the women's age, the percentage of normal embryos and fertility status of the couple are presented in Tables 3, 4 and 5.
The male carriers had 147 cycles with OPU and in 70 of these ET could take place leading to 24 ongoing pregnancies. For the female carriers, 165 cycles with OPU were performed with ET in 80, giving rise to 16 ongoing pregnancies.
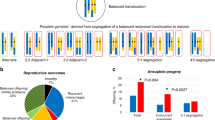
In total, 312 cycles with OPU were performed (median of 2 cycles per couple, range 1–6) and a median of 12 cumulus oocyte complexes (range 2–32) was retrieved per cycle. Out of a total of 3895 cumulus oocyte complexes, 60.5% fertilized normally and 41.7% were biopsied. In 3.9% of the biopsied blastomeres, no FISH analysis could be done because of the presence of no, multiple or fragmented nuclei. FISH was attempted on 1563 blastomeres and 1553 (99.4%) had a diagnosis of which 323 (20.8%) had a normal test result; no significant difference was found between female and male carriers (19.2% and 22.5%, respectively).
It was not the aim of this study to look at the translocation segregation modes in the embryos and hence no such data are provided here.
In 82/312 (26.3%) cycles aneuploidy testing for chromosomes 13,18,21,X and Y was additionally performed on 141 embryos. An abnormal result was found in 66 (46.8%) of these embryos.
ET was not possible in 162/312 (51.9%) cycles and this because – with regard to the parental translocation – in 82.7% of these cycles all embryos had an abnormal test result. In 14.2% of the cycles without ET poor embryo morphology was responsible for ET not being possible and in 3.1% aneuploidy testing indicated chromosome aneuploidy in an otherwise transferable embryo.
ET was feasible in 150 cycles (48.1% of the cycles with OPU) and positive hCG was found in 36.7% of these cycles.
The live-born delivery rate was 12.8% per cycle with OPU and 26.7% per cycle with ET.
For couples with a positive hCG, the live-born delivery rate was 72.7% (40/55). When aneuploidy screening for chromosomes 13, 18, 21, X and Y had been performed, 68.8% (11/16) of the positive hCG eventually led to delivery of one or more healthy children, and without aneuploidy screening, this figure was 74.4% (29/39).
For male carriers, a lower success rate is seen when the spouse is more than 35 years at the moment of intake (see Table 3) compared with the spouse who is less than 35 years. However, this difference is not yet statistically significant, probably because of the limited number of patients. Indeed, when we look at the number of embryos with normal FISH result compared with the total number of embryos with diagnosis, the women less than 35years have a significantly higher number of normal embryos (OR 1.91, CI 1.12-3.29). This female age effect was not observed for female carriers.
The outcome of the PGD treatment was not statistically different for cycles with less or more than 50% normal embryos (Table 4). However, when more than one balanced/normal embryo was available, the couple had a better chance of having a live born child at the end of that cycle. This is logical as then the best quality embryo can be selected.
Fertile couples undergoing PGD have no better treatment outcome compared with the infertile PGD couples (Table 5).
The median for the time between the finishing of the PGD work-up and the delivery of the couples’ first PGD child was 15.0 months (range 11–76 months) for male carriers and 15.5 months (range 9–36 months) for female carriers.
Children
A total of 47 children were born from 33 singleton and 7 twin deliveries. Data on mean gestational age and birth parameters are presented in Table 6. One child had neurodevelopmental delay, and work-up revealed cerebellar vermis hypoplasia and mega cisterna magna. This child would now be a good candidate for microarray analysis to detect submicroscopic deletions or duplications, but this was not available at the time of follow-up. No other major malformations were registered in the children. The karyotype of 37/47 children was known by means of prenatal (17) or postnatal (20) testing. The translocation was inherited in 18 children and 18 had a normal karyotype with regard to the translocation. One child without the translocation had a numerical chromosomal anomaly, namely 47,XYY. He was the child of a male rcp carrier and no aneuploidy testing had been performed in the cycle leading to the birth of this child.
Discussion
Similar to the study on PGD for Robertsonian translocations,18 the majority of the couples in the present paper are not Belgian residents. This reflects the fact that PGD is not ubiquitously available and that couples are willing to travel to fulfill their child wish. The reasons why almost half of the couples eventually did not proceed with PGD are diverse, but it should be noticed that the technically unfeasible PGDs date from the early days of the procedure. A substantial part of the ‘request-only group’ had a spontaneous pregnancy or used other assisted reproduction techniques (without PGD) and this shows that people frequently try different reproductive options in parallel to see what is the fastest way to fulfil their desire to have a child. From the couples’ point of view, this is not surprising as the female age at intake was already higher than the mean maternal age for primiparae (27.6 years) and multiparae (30.5 years) in Flanders (figures from 2006, www.zorg-en-gezondheid.be/cijfers/cijfers-over-geboorte-en-bevalling) and they had been trying for a child for several years. However, work-up of PGD tests that are subsequently not required, is a waste of limited laboratory resources.
In contrast to PGD for monogenic disorders, where moral and ethical considerations are also important reasons to opt for PGD,19 couples with rcp almost exclusively choose PGD because of fertility problems or repeated miscarriages. This bias is inherent to the problems induced by carriership of translocations. The reproductive history of the couples, before their PGD treatment, was mostly unsuccessful with a live-born delivery rate of 4.8% and 9.7% for the male and female carriers, respectively. Moreover, a substantial subset of these children had congenital anomalies secondary to unbalanced inheritance of the parental translocation. In contrast to these low figures, the present study showed a live-born delivery rate of 16.3% per cycle with OPU and of 34.3% per cycle with ET in male rcp carriers, and of 9.7% and 20%, respectively, in female carriers. In the study population, PGD was able to dramatically increase the live-born delivery rate up to 59.3% for female carriers and up to 85.7% in male carriers once there was a positive hCG. This means that the miscarriage rate in PGD pregnancies was similar to that in the general population.20 No statistically significant difference in the miscarriage rate was observed between cycles with or without additional aneuploidy screening for chromosomes 13, 18, 21, X and Y.
As expected, ET was cancelled in a high number of cycles because no chromosomally normal embryos were available. However, once ET could take place, delivery rate is comparable to that seen in PGD for other indications in our center.12, 18
The results are also comparable to those of Ko et al21 and the same trend is observed by Fischer et al.22 The present figures are also in line with those from the ESHRE PGD Consortium data collection IX and X16, 23 where for male carriers a delivery rate of 12–13% per OPU and 22% per ET was found and for female carriers 13–14% per OPU and 24%–26% per ET.
Fecundity is a complex issue, and the sum of male and female factors. In couples with a rcp carrier, it can be assumed that the translocation has an important role in the decline of the couples’ fertility. When a couple encounters reproductive problems because of the rcp in the woman, it could be hypothesized that the additional effect of her age on the (in)fertility of the couple is small. If, however, the male carries the rcp and the woman is over 35years, this extra female factor could be relatively more important, thus leading to lower success rate of PGD. This hypothetical explanation remains difficult to prove because it would need a comparative study with couples matched for age, sperm parameters and ovarian function.
Nevertheless, the observed difference between the groups, might help to give couples a realistic perspective with regard to their PGD treatment.
With regard to the birth parameters of children born after PGD for rcp, the observed data are comparable to those of children born after PGD for Robertsonian translocation18 and of children born after ICSI.24 The incidence of major congenital malformations was 1/47 (2.12%) which is in line with the series of Desmyttere et al25 and with the Eurocat figure of 23.9/1000.26
The success figures of PGD should also be compared with what is known on the outcome of natural conceptions in fertile carriers. Sugiura-Ogasawara et al27 found that of 72 rcp carriers, ascertained by recurrent miscarriage, 29 (40.3%) experienced the birth of a healthy child after a mean of 10.1±7.7 months. However, 26 (36.1%) did not get pregnant in that time frame and 17 (23.6%) had another miscarriage. For the successful PGD couples, their first PGD child was mostly born within 1.5 years after the work-up for PGD. This is only half of the time they had the desire for a child before they came to our center.
Additional aneuploidy screening for chromosomes not involved in the translocation did not improve the results of PGD in this study. This is in line with the findings of Gianaroli et al28 who found common aneuploidies in only 6% of the embryos that were normal for the translocation chromosomes.
Carriers should be encouraged to inform their relatives, so that early carrier testing can be offered and thus precious reproductive time can be gained.
Taking into account the results of the present study and data from the literature, we conclude that PGD is a reasonable reproductive option for infertile couples with a rcp carrier as despite their sub- or infertility they have equal chances of having a child by PGD compared with the fertile carrier couples. However, even the latter group can sometimes benefit from PGD treatment, especially when the grief caused by subsequent miscarriages becomes a major burden. Besides, couples experiencing time pressure because of (maternal) age could also consider PGD, but male carriers with women above 35years should be warned that their chances could be low.
Large prospective studies are needed to further improve counseling of translocation carriers, but meanwhile all reproductive options – including PGD – should be discussed with them so that they can make informed choices.
References
Iselius L, Lindsten J, Aurias A et al: The 11q;22q translocation: A collaborative study of 20 new cases and analysis of 110 families. Hum Genet 1983; 64: 343–355.
Kurahashi H, Inagaki H, Ohye T, Kogo H, Kato T, Emanuel BS : Chromosomal translocations mediated by palindromic DNA. Cell Cycle 2006; 5: 1297–1303.
Hamerton JL, Canning N, Ray M, Smith S : A cytogenetic survey of 14,069 newborn infants. Incidence of chromosome abnormalities. Clin Genet 1975; 8: 223–243.
Van Dyke DL, Weiss L, Robertson JR, Babu VR : The frequency and mutation rate of balanced autosomal rearrangements in man estimated from prenatal genetic studies for advanced maternal age. Am J Hum Genet 1983; 35: 301–308.
Fryns JP, Van Buggenhout G : Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol 1998; 81: 171–176.
Meza-Espinoza JP, Anguiano LO, Rivera H : Chromosomal abnormalities in couples with reproductive disorders. Gynecol Obstet Invest. 2008; 66: 237–240.
Nussbaum RR, McInnes RR, Willard HF : Principles of clinical cytogenetics; in Thompson & Thompson Genetics in Medicine. Philadelphia: Saunders, 2007, 7th edn, pp 74–75.
Ogilvie CM, Braude P, Scriven PN : Successful pregnancy outcomes after preimplantation genetic diagnosis (PGD) for carriers of chromosome translocations. Hum Fertil (Camb.) 2001; 4: 168–171.
Harton GL, Harper JC, Coonen E, Pehlivan T, Vesela K, Wilton L : European Society for Human Reproduction and Embryology (ESHRE) PGD Consortium. ESHRE PGD consortium best practice guidelines for fluorescence in situ hybridization-based PGD. Hum Reprod 2011; 26: 25–32.
Vandervorst M, Liebaers I, Sermon K et al: Successful preimplantation genetic diagnosis is related to the number of available cumulus-oocyte complexes. Hum Reprod 1998; 13: 3169–3176.
Devroey P, Van Steirteghem A : A review of ten years experience of ICSI. Hum Reprod Update 2004; 10: 19–28.
Verpoest W, Haentjens P, De Rycke M et al: Cumulative reproductive outcome after preimplantation genetic diagnosis: a report on 1498 couples. Hum Reprod 2009; 24: 2951–2959.
De Vos A, Van Steirteghem A : Aspects of biopsy procedures prior to preimplantation genetic diagnosis. Prenat Diagn 2001; 21: 767–780.
Staessen C, Coonen E, Van Assche E et al: Preimplantation diagnosis for X and Y normality in embryos from three Klinefelter patients. Hum Reprod 1996; 11: 1650–1653.
Staessen C, Tournaye H, Van Assche E et al: PGD in 47,XXY Klinefelter's syndrome patients. Hum Reprod Update 2003; 9: 319–330.
Harper JC, Coonen E, De Rycke M et al: ESHRE PGD Consortium data collection X: cycles from January to December 2007 with pregnancy follow-up to October 2008. Hum Reprod 2010; 25: 2685–2707.
Bonduelle M, Liebaers I, Deketelaere V et al: Neonatal data on a cohort of 2889 infants born after ICSI (1991–1999) and of 2995 infants born after IVF (1983–1999). Hum Reprod. 2002; 17: 671–694.
Keymolen K, Staessen C, Verpoest W et al: A proposal for reproductive counselling in carriers of Robertsonian translocations: 10 years of experience with preimplantation genetic diagnosis. Hum Reprod 2009; 24: 2365–2371.
De Rademaeker M, Verpoest W, De Rycke M et al: Preimplantation genetic diagnosis for myotonic dystrophy type 1: upon request to child. Eur J Hum Genet 2009; 17: 1403–1410.
Buss L, Tolstrup J, Munk C et al: Spontaneous abortion: a prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. Acta Obstet Gynecol Scand. 2006; 85: 467–475.
Ko DS, Cho JW, Park SY et al: Clinical outcomes of Preimplantation Genetic Diagnosis (PGD) and Analysis of Meiotic Segregation Modes in Reciprocal Translocation Carriers. Am J Med Genet Part A 2010; 152A: 1428–1433.
Fischer J, Colls P, Escudero T, Munné S : Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril 2010; 94: 283–289.
Goossens V, Harton G, Mouton C, Traeger-Synodinos J, Van Rij M, Harper J : ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod 2009; 24: 1786–1810.
Bonduelle M, Wennerholm UB, Loft A et al: A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod 2005; 20: 413–419.
Desmyttere S, Verpoest W, De Rycke M et al: Neonatal outcome of 995 children conceived after embryo biopsy compared to children born after intracytoplasmic sperm injection. Hum Reprod 2010; 25 (Suppl 1): 2945–2950.
Dolk H, Loane M, Garne E : The prevalence of congenital anomalies in Europe. Adv Exp Med Biol 2010; 686: 349–364.
Sugiura-Ogasawara M, Aoki K, Fujii T et al: Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J Hum Genet 2008; 53: 622–628.
Gianaroli L, Magli MC, Ferraretti AP et al: Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod 2002; 17: 3201–3207.
Acknowledgements
This study was supported by a research grant from the University Hospital UZ Brussel VUB, University Research Council, FWO Vlaanderen, Willy Gepts Foundation, EU funding, Bertarelli Foundation, Shering-Plough International and Belgium, Merck Sharp and Dohme Belgium, IBSA and Ferring International.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Keymolen, K., Staessen, C., Verpoest, W. et al. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur J Hum Genet 20, 376–380 (2012). https://doi.org/10.1038/ejhg.2011.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2011.208
Keywords
This article is cited by
-
Conventional ICSI improves the euploid embryo rate in male reciprocal translocation carriers
Journal of Assisted Reproduction and Genetics (2021)
-
Pregnancy outcomes of reciprocal translocation carriers with two or more unfavorable pregnancy histories: before and after preimplantation genetic testing
Journal of Assisted Reproduction and Genetics (2019)
-
Reproductive outcomes following preimplantation genetic diagnosis using fluorescence in situ hybridization for 52 translocation carrier couples with a history of recurrent pregnancy loss
Journal of Human Genetics (2016)
-
Genetic counseling for men with recurrent pregnancy loss or recurrent implantation failure due to abnormal sperm chromosomal aneuploidy
Journal of Assisted Reproduction and Genetics (2016)
-
Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study
European Journal of Human Genetics (2013)