Abstract
The first GalT gene knockout (KO) mouse model for Classic Galactosemia (OMIM 230400) accumulated some galactose and its metabolites upon galactose challenge, but was seemingly fertile and symptom free. Here we constructed a new GalT gene-trapped mouse model by injecting GalT gene-trapped mouse embryonic stem cells into blastocysts, which were later implanted into pseudo-pregnant females. High percentage GalT gene-trapped chimera obtained were used to generate heterozygous and subsequently, homozygous GalT gene-trapped mice. Biochemical assays confirmed total absence of galactose-1 phosphate uridylyltransferase (GALT) activity in the homozygotes. Although the homozygous GalT gene-trapped females could conceive and give birth when fed with normal chow, they had smaller litter size (P=0.02) and longer time-to-pregnancy (P=0.013) than their wild-type littermates. Follicle-stimulating hormone levels of the mutant female mice were not significantly different from the age-matched, wild-type females, but histological examination of the ovaries revealed fewer follicles in the homozygous mutants (P=0.007). Administration of a high-galactose (40% w/w) diet to lactating homozygous GalT gene-trapped females led to lethality in over 70% of the homozygous GalT gene-trapped pups before weaning. Cerebral edema, abnormal changes in the Purkinje and the outer granular cell layers of the cerebellum, as well as lower blood GSH/GSSG ratio were identified in the galactose-intoxicated pups. Finally, reduced growth was observed in GalT gene-trapped pups fed with normal chow and all pups fed with high-galactose (20% w/w) diet. This new mouse model presents several of the complications of Classic Galactosemia and will be useful to investigate pathogenesis and new therapies.
Similar content being viewed by others
Introduction
Classic Galactosemia (OMIM 230400) is an autosomal recessive disorder caused by deficiency of galactose-1 phosphate uridylyltransferase (GALT, EC 2.7.7.12) activity (Supplementary Figure 1).1, 2, 3, 4, 5 GALT is the second enzyme in the evolutionarily conserved galactose metabolic pathway, and facilitates the simultaneous conversion of uridine diphosphoglucose and galactose-1 phosphate (gal-1P) to uridine diphosphogalactose (UDP-galactose) and glucose-1 phosphate (Supplementary Figure 1).6 GALT deficiency leads to accumulation of gal-1P, deficiency of UDP-galactose and other metabolic derangements.7, 8 If untreated, Classic Galactosemia can be lethal for the affected newborns.1, 5 Since inclusion of this disease in the newborn screening panel in the US, neonatal mortality has decreased.9 The mainstay of treatment is the withdrawal of galactose from the diet.5 Despite early dietary management, chronic complications such as intellectual deficits, ataxia, speech dyspraxia, premature ovarian insufficiency (POI) and decreased bone mineralization occur in many adults.10, 11, 12, 13, 14, 15, 16, 17, 18, 19
Different cell and animal models have been utilized to understand the pathogenic mechanisms of the acute neonatal lethality and the long-term complications associated with Classic Galactosemia. Early genetic studies in the yeast Saccharomyces cerevisiae showed that GALT-deficient mutant yeasts are sensitive to galactose in growth medium; but disruption of galactokinase (GALK) function in these yeasts reversed their galactose sensitivity.20, 21 This seminal work re-affirmed the suspected pathogenic role of gal-1P, product of GALK, in GALT-deficiency, but did not reveal the mechanism of the in vivo toxicity of gal-1P leading to speech dyspraxia and POI. The first GalT gene-knockout (KO) mouse model for Classic Galactosemia22, 23, 24 had moderate accumulation of galactose and gal-1P upon galactose challenge, but no overt human disease phenotypes.22, 23, 24 The first dGALT-deficient Drosophila melanogaster model had arrested larval development with a high-galactose diet.25 Impaired geotaxic response was also seen in these flies despite dietary restriction of galactose.25 However, there were no reports on reduced fertility in these fruit flies and more importantly, the metamorphic changes in the life cycle of this insect are non-existent in humans, making it difficult to study any potential pre-natal effects of galactose toxicity in human patients.
In this study, we constructed a new GalT gene-trapped mouse model. These new GalT gene-trapped mice, similar to the previous GalT-KO mice, showed some degrees of resistance to galactose toxicity, but expanded characterization revealed subtle phenotypic differences between the mutant mice and their wild-type (ie, normal) littermates, which could shed new insights into the pathophysiology of the disease.
Materials and methods
Targeting GalT gene in murine embryonic stem (ES) cells and construction of the GalT gene-trapped mice
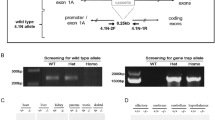
E285B04 gene trap ES cells with GalT gene trap were produced by the German Genetrap Consortium (GGTC) and were obtained under an official Materials Transfer Agreement between GGTC and the University of Utah. The GalT gene was trapped using VelociGene’s COMP Definitive Null Allele Design, which deletes the GalT gene by replacing critical exons with a reporter/selection cassette. Gene-trapped ES cells are heterozygous for the null mutation. ES cells were grown, prepared and injected at the University of Utah Transgenic and Gene Targeting Mouse Core. In all, 12–15 ES cells, derived from agouti 129/Ola mice, were injected into the blastocoel of black C57Bl/6J e3.5 day blastocysts to generate agouti and black chimeras. Injected blastocysts were surgically implanted into e2.5 day pseudo-pregnant CBA × C57Bl/6J F1 females and allowed to progress naturally to birth and weaning. High percent chimeric males were crossed to C57Bl/6J females to ascertain germline transmission. All agouti offspring were genotyped to determine the presence of the heterozygous allele. As expected, approximately 50% of the agouti offspring were heterozygous for the gene trap allele (Figure 1a). Heterozygous mice were intercrossed to produce offspring that were homozygous for the mutant allele, hence were deficient in GalT. Expected Mendelian ratios of wild-type, heterozygotes and homozygous null mutants were obtained. All mice are maintained in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines, and protocols approved by the Institutional Animal Care and Use committee are strictly followed at all times.
Construction and biochemical characterization of a new GalT gene-trapped mouse model. (a) Schematic of GalT gene-trapped mouse model construction and molecular genotyping of the GalT gene trap allele. (b) Red blood cell GALT activity was measured in 10-day-old mice with various GALT genotypes by a LC-MS/MS method described by Li et al.26 (c) Gal-1P contents in RBC lysate of mice fed with normal chow/high-galactose diet were measured by enzymatic method previously described.8
Molecular genotyping of the GalT gene trap allele
DNA from tail clips was harvested by alkaline hydrolysis and the presence of the GalT gene trap was confirmed using PCR primer Splirev2 (5′-GCCAAACCTACAGGTGGGGTCTTT-3′) and E285 (5′-CCAAGCTCAGGTCTTCGTCT-3′). Wild-type GalT allele was amplified with PCR primers SU (5′-CGGTGTGCAGGAGAATCATC-3′) and E285 (CCAAGCTCAGGTCTTCGTCT-3′). PCR conditions for both wild-type and gene-trapped alleles were set as follows: (1) initial denaturing step: 94 °C for 2 min; (2) 36 cycles of amplification steps comprising of denaturing at 94 °C for 30 s, annealing at 56 °C for 30 s and elongation at 72 °C for 90 s; and (3) final elongation step at 72 °C for 5 min.
Galactose challenge of mice
Modified RMH with 40% (or 20%) galactose diet was manufactured by Test Diet Inc. (Richmond, IN, USA).
Biochemical studies
Determination of gal-1P was performed using methods described previously.8 GALT activity was determined according to procedure published by Li et al,26 which used a direct method with LC-MS/MS detection of the product of the reaction. Waters XBridge BEH amide column (2.1 mm × 100 mm, 3.5 μm) and AB Sciex API 4000 system (AB Sciex IS, Framingham, MA, USA) equipped with Shimadzu LC units was used in this study.
Determination of glutathione levels
Blood GSH and GSSG levels were determined by the 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB) assay according to the previous reported method27 with a minor modification. Briefly, whole blood was kept in cold metaphosphoric acid (5%) buffer after the blood draw. Red blood cells were lysed by vigorous vortexing and then the mixture was centrifuged at 10 000 × g for 10 min before the supernatant was transferred to new tubes for further assay. The reaction mixture contained 1 mM EDTA, NADPH (0.24 mM), glutathione reductase (0.06 U), DTNB (86 μ M) and samples. Yellow 5-thio-2-nitrobenzoic acid formation was monitored at 412 nm. GSSG was measured by masking the GSH in the metaphosphoric acid (5%) buffer with 10 mM 4-vinylpyridine in the presence of 6% triethanolamine and derivatized at 37 °C overnight.
Histological studies
Organs harvested immediately from killed animals were fixed in 10% formalin for at least 48 h before sending off to the histology research core at ARUP Laboratories (Salt Lake City, UT, USA) for tissue processing and slide preparations. H&E-stained sections prepared were sent off to board-certified pathologists at University of Utah and University of Florida, respectively, for analysis in a single-blinded manner at which the pathologists were unaware of the genotypes of the mice until the results were reported. For estimation of the number of ovarian follicles, at least three slide sections (50 μ M apart) were prepared from each ovary from each female mouse.
Statistical analysis
If required and appropriate, Student’s T-test (two-tailed) was performed to evaluate whether differences between two groups are significant.
Hematocrit determination
Volumes of red blood cells were determined from 40 to 45 μl of blood drawn from WT and homozygous GalT gene-trapped females at different time points after galactose (40% w/w) challenge. Values obtained were expressed as percentages of the total blood volumes in the respective samples.
Results
In this study, we developed a new GalT gene-trapped mouse model to test the in vivo efficacy of a novel therapy aimed to reduce the accumulation of toxic level of gal-1P in Classic Galactosemia.
Molecular genotypes of the new GalT gene-trapped mouse models
Using PCR-based methods, we confirmed the presence of the GalT gene trap alleles in mice, which are either heterozygous or homozygous for the allele (Figure 1a).
Biochemical phenotypes of the new GalT gene-trapped mouse model
Using the LC-MS/MS procedure published by Li et al,26 we confirmed the total absence of GALT activity in the homozygous GalT gene-trapped mice (Figure 1b). Also, the homozygous GalT gene-trapped mice accumulated up to 60.8±27.3 μ M of gal-1P in RBC even when they were unchallenged (ie, fed with normal chow; Figure 1c). However, when these animals were fed with high-galactose (40% w/w) diet (Test Diet Inc.), their blood gal-1P increased only to 432±31 μ M, which was lower than that reported in the untreated/undiagnosed human newborns.5
Galactose challenge in GalT gene-trapped lactating females led to lethality in newborn GalT gene-trapped mice
Newborns with Classic Galactosemia usually die shortly after birth if they receive normal human milk. Mouse milk contains 66% less lactose than human milk,28 but the previous GalT-KO pups were never challenged with excess galactose before they were weaned to mimic the high galactose load of human milk.22 Therefore, those mice could have survived because of the minimal challenge received. It is technically difficult and invasive to force feed the newborn pups with (mouse) milk containing added galactose, but it is reasonable to assume that the excess dietary galactose, which cannot be properly metabolized by nursing homozygous GalT gene-trapped mothers, be passed to their newborn pups through milk.29, 30, 31, 32, 33 Therefore, we fed the lactating homozygous GalT gene-trapped mothers with high-galactose (40% w/w) diets as soon as the homozygous GalT gene-trapped pups were born. Over 70% of the newborn pups of the galactose-challenged mothers died before weaning (row 3, Table 1). Such high levels of lethality were not seen when wild-type mothers were fed with excess dietary galactose (row 5, Table 1). Neither did we observe the same extent of lethality resulting from heterozygous pups nursed by homozygous GalT gene-trapped mothers fed with high galactose (row 4, Table 1). Biochemical analysis of livers harvested from the succumbed GalT gene-trapped newborns confirmed the presence of large amounts of gal-1P (∼20 μg gal-1P per mg liver cell protein), and histological examination excluded signs of starvation in the liver, such as accumulation of periportal fat. Thus, our observations confirmed that GALT deficiency in both the mother and the newborns was necessary to observe the acute lethal phenotype induced by galactose challenge of the mother.
Galactose challenge in GalT gene-trapped lactating females affects the brains of the newborn GalT gene-trapped mice
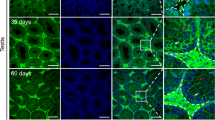
Histological examination of the succumbed pups reveal mild inflammatory responses in the livers (data not shown), but there were no overt signs of sepsis or bleeding disorder. Yet, the brains of the intoxicated pups showed increased inflammatory response in the cerebrum (Figure 2a). The Purkinje cells appeared smaller, more acidophilic and the cell layer seemed to be punctate (Figures 2b and c). The outer granular cell layer of the cerebellum was also thicker in the intoxicated animals (Figure 2d).
Histological examination of brains of galactose-intoxicated homozygous GalT gene-trapped (G/G) mice. Left panels showed the brain sections of the galactose-intoxicated homozygous GalT gene-trapped (G/G) mice and the right panel showed the same area of the brain obtained from G/G mice, which were not intoxicated. (a) Infiltration of lymphocytes in cerebrum (arrows, left panel; H&E, × 40). (b) Smaller Purkinje cells with larger gaps (arrows) were seen in the cerebellum (left panel; H&E, × 40). (c) Extensive acidophilic changes in the Purkinje cells (arrows, left panel; H&E, × 40). (d) Thicker outer granular cell layers were seen in the cerebellum (circled, left panel; H&E, × 40).
Galactose challenge in GalT gene-trapped lactating females causes oxidative stress in newborn GalT gene-trapped mice
A recent study using Drosophila as an animal model of GALT deficiency suggested that oxidative stress has a significant role in the toxicity syndrome seen in this model.34 To assess oxidative stress in the galactose-intoxicated pups, we measured the GSH/GSSG ratio in the blood of the animals (Supplementary Figure 2). In the absence of galactose challenge of the mothers, there was no difference in the blood GSH/GSSG ratio between wild-type and homozygous GalT gene-trapped pups. However, the mutant pups exhibited a lower GSH/GSSG ratio when they were challenged via the mothers’ milk, suggesting that excess galactose led to manifestation of oxidative stress in these pups.
Growth restriction in GalT gene-trapped mice
If the intoxicated pups survived the toxic insult of galactose, they manifested severe growth restriction later in life, even when normal chow resumed after the weaning period ended (Figures 3a and b).35 As 40% (w/w) galactose diet induced significant deaths in newborn homozygous GalT gene-trapped pups, it was difficult for us to study the long-term effect of galactose challenge on the growth of these mice. When galactose levels in the diet of the nursing mothers were reduced to 20% (w/w), the survival of the pups increased to 100%. Figure 3c shows the growth of normal (wild-type) and homozygous GalT gene-trapped mice up to 10 weeks of age. Both groups of mice were exposed to either normal chow or 20% galactose diet. The normal (wild-type) mice attained the higher average growth rate and maximum weight when compared with their homozygous GalT gene-trapped counterparts, even when both groups were unchallenged and fed with regular diets.
Effect of galactose on growth in mice with different genotypes. (a) A 9-day-old (smaller) homozygous GalT gene-trapped pup from a galactose-challenged lactating mother posed next to a 9-day-old homozygous GalT gene-trapped pup (bigger) whose mother was not fed with a high-galactose diet. (b) A 46-day-old homozygous GalT gene-trapped mouse (smaller) who survived galactose challenge during the nursing period posed next to an age-matched homozygous GalT gene-trapped pup mouse whose mother did not receive any galactose challenge. Galactose challenge of the surviving mouse stopped after day 21, the end of the weaning period. (c) Body weights of normal, wild-type (N/N) and homozygous GalT gene-trapped (G/G) mice were measured for a period of about 10 weeks since birth. Before weaning, half of the G/G mice were nursed by homozygous GalT gene-trapped mothers (red crosses) challenged with 20% galactose-diet and half were nursed by homozygous GalT gene-trapped mothers fed with normal chow (red diamonds). After weaning, the pups exposed to 20% galactose were fed with the same diet while the pups exposed to normal chow continued to be fed with normal diet. Similar treatments were rendered for the wild-type (N/N) mice nursed by N/N mothers. Each data points represented the average of six mice and the error bars represented ±1 SD.
Surprisingly, the growth rate and weight of both the wild-type and the homozygous GalT gene-trapped mice were reduced when they were exposed to 20% galactose diets since birth. These results show that postnatal exposure to high galactose can induce growth restriction in the homozygous GalT gene-trapped mice. In addition, since growth reduction also occurred in the wild-type mice, which were able to utilize galactose, suggesting that growth reduction in the homozygous GalT gene-trapped mice cannot solely be explained by their inability to utilize the galactose in the food and thus, received less calories/nutrients.
Although the homozygous GalT gene-trapped fed with 20% galactose initially trailed slightly behind the homozygous GalT gene-trapped mice fed with normal chow in their rate of growth, they finally caught up at about day 50, which marked the onset of puberty. It appears that these GALT-deficient mice significantly overcame their growth sensitivity to galactose at this stage of their life cycle. However, such reversal of growth sensitivity was not immediately seen in the wild-type mice fed with 20% galactose.
Reduced fertility in the GalT gene-trapped female mice
In sharp contrast to human female patients with Classic Galactosemia who are on a galactose-restricted diet, the female homozygous GalT gene-trapped mice appear to be fertile when they were fed with normal chow. However, when we examined these mice over a long period of time, we observed that these mice had significantly smaller litter size (P=0.02; Figure 4a) and significantly longer time-to-pregnancy (P=0.013; Figure 4b) than their wild-type littermates, suggesting diminished reproductive capacity, also known as subfertility in the homozygotes.36 For instance, in spite of normal chow, the GALT-deficient mice had a maximum of three litters throughout their life (Figure 4b). Levels of follicle-stimulating hormone were not different between age-matched mutant and wild-type females (data not shown). Yet, histological examination of ovaries dissected from age-matched mutant females fed with normal chow consistently showed fewer numbers of follicles per ovarian section than their wild-type counterparts (Figures 4c and d; P=0.0073), despite follicles at different stages of development were seen. Ovaries of the homozygous GalT gene-trapped mice also had more corpora lutea of larger size than the ovaries of wild-type females (Figure 4c).
Subfertility phenotypes in unchallenged homozygous GalT gene-trapped (G/G) female mice. (a) Average litter size was calculated for females with different genotypes. Error bars represent ±1 SD. (b) Time to pregnancy (TTP) of GALT-deficient (G/G) mice and wild-type mice (n=4 for both) were compared. TTP was counted from the birth date of the previous litter to the birth date of the next litter. G/G #1 did not conceive for >180 days after her third pregnancy and G/G #3 had only one pregnancy throughout the study period. (c) H&E sections of ovaries from three G/G mice of 6 months of age (upper panel) and three age-matched wild-type mice (lower panel) ( × 5 magnification) (CL, corpus luteum). (d) Follicle counts from 8 wild-type and 11 G/G females fed with normal chow.
Discussion
Originally constructed for the purpose of assessing the in vivo efficacy of novel therapies for Classic Galactosemia, the new GalT gene-trapped mouse model described here exhibited phenotypes not reported in the original GalT-KO mouse model:22
-
a)
galactose challenge of the homozygous GalT gene-trapped newborns led to high mortality rate (Table 1), insults in brain tissues (Figure 2) and altered GSH/GSSG ratio in red blood cells (Supplementary Figure 2);
-
b)
growth restriction in non-challenged homozygous GalT gene-trapped mice (Figure 3); and
-
c)
reduced fertility in non-challenged homozygous GalT gene-trapped females (Figure 4).
How do these findings compare with other cell/animal models of GALT deficiency and patients with Classic Galactosemia? The excess mortality among the intoxicated mouse pups is in-line with the galactose sensitivity phenotype seen in galactose-intoxicated gal7-deleted (GALT-deficient) yeast,37, 38 dGALT-null (GALT-deficient) fruitflies and human neonates.25 Of course, it does not necessarily mean that the toxicity mechanisms involved are identical in all cases. At the moment, the cause of death for the intoxicated pups remains unclear, despite the fact that oxidative stress had a pathogenic role in the galactose-induced lethality in the GALT-less Drosophila model of Classic Galactosemia.34 Without further studies involving anti-oxidants supplementation, we cannot be certain whether the degree of oxidative stress experienced by these pups led to the lethal consequences, nor do we know how excess galactose caused oxidative stress in these pups. Alternatively, high concentration of galactose contained in the dam’s milk could also lead to osmotic stress, but histological examination of the tissues of the dead pups did not support this hypothesis (data not shown). Moreover, we had monitored the hematocrit values of galactose-challenged homozygous GalT gene-trapped female mice and found that short term (up to 18 days) of galactose challenge did not result in dehydration (ie, increase in hematocrit; Supplementary Figure 3).
As we did not feed the newborn pups directly with galactose, we were concerned if galactose challenge of the lactating females, especially those homozygous for the GalT gene-trapped alleles, may render them sick and change their feeding behavior. But similar to what Leslie et al22 reported, galactose challenge of the adult homozygous GalT gene-trapped mice did not have short-term overt adverse effects, nor did we observe any changes in (nursing) behavior that may have contributed to the negligence or starvation of the newborn pups. In fact, we noticed that the stomachs of the succumbed homozygous GalT gene-trapped pups were often full of milk (Supplementary Figure 4), emphasizing that it was not the lack of nursing that caused their deaths. The accumulation of high level of galactose metabolites, as well as absence of signs of starvation in the liver tissues of the succumbed pups, was another indication that the pups were well-fed and galactose present in the mother’s diet was passed to the pups via milk.
The brain insult seen in the succumbed pups has never been reported among all animal models and could have significant implications in the pathophysiology of the ataxia phenotype seen in some human patients with galactosemia. The changes in Purkinje cells, which occurred only in the galactose-intoxicated pups, also suggested a post-natal role of galactose in the central nervous system pathology.
The non-challenged homozygous GalT gene-trapped mice had growth restriction (Figure 3) and reduced fertility (Figure 4), phenotypes that resemble those seen in human patients. Our mouse model thus confirms the importance of GALT activity in ovarian function, independently from other genes or exogenous factors that confounded the previous experimental approaches of experimental hypergalactosemia39, 40 and artificial activation of the prolactin short form receptor in the ovary.41
As for the absence of sepsis and bleeding disorder in the intoxicated newborn pups, there is serotype specificity of the Escherichia coli involved in human sepsis42, 43 and this specific bacterial species may be absent in the normal flora of the murine species. The lack of bleeding disorder, which was previously thought to be predominantly due to liver failure, may also be explained by inter-species differences in glycosylation between humans and mice.44, 45, 46 Nevertheless, most animal models of human diseases do not recapitulate all aspects of the human pathology. For example, the early mouse model of cystic fibrosis never acquired spontaneous and chronic lung infections, but manifested meconium ileus, alteration of mucous and serous glands, and obstruction of gland-like structures.47 Using the strategy of conditional KO, Hodges et al48 finally confirmed the role played by cystic fibrosis transmembrane conductance regulator in lung inflammation in mice.
Despite the above-mentioned differences, there are similarities between the old GalT-KO and the new GalT gene-trapped mouse models. Female mice were able to conceive and give birth at young age; and the adult mice were relatively resistant to galactose toxicity in both cases. In addition, like the original GalT-KO model, the new mouse model accumulated only modest level of gal-1P in red blood cells, even under galactose challenge (Figure 1c). The molecular mechanisms that underlie the lower accumulation of gal-1P in these GALT-deficient mice are unknown, but they may explain the higher galactose resistance in these rodents compared with humans.
References
Isselbacher KJ, Anderson EP, Kurahashi K, Kalckar HM : Congenital galactosemia, a single enzymatic block in galactose metabolism. Science 1956; 123: 635–636.
Fridovich-Keil JL : Galactosemia: the good, the bad, and the unknown. J Cell Physiol 2006; 209: 701–705.
Tyfield L, Reichardt J, Fridovich-Keil J et al: Classical galactosemia and mutations at the galactose-1-phosphate uridyl transferase (GALT) gene. Hum Mutat 1999; 13: 417–430.
Berry GT, Segal S, Gitzelmann R : Disorders of galactose metabolism; in: Fernandes J, Saudubray M, van den Berghe G, Walter JH (eds): Inborn Metabolic Diseases - Diagnosis and Treatment. Springer-Verlag: New York, 2006 4th edn.
Segal S, Berry GT : Disorders of galactose metabolism; in: Scriver CR, Beaudet AL, Sly WS, Valle D (eds): The Metabolic Basis of Inherited Diseases. McGraw-Hill: New York, 1995; Vol I: pp 967–1000.
Leloir LF : The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem 1951; 33: 186–190.
Gitzelmann R : Galactose-1-phosphate in the pathophysiology of galactosemia. Eur J Pediatr 1995; 154: S45–S49.
Lai K, Langley SD, Khwaja FW, Schmitt EW, Elsas LJ : GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 2003; 13: 285–294.
Kaye CI, Accurso F, La Franchi S et al: Newborn screening fact sheets. Pediatrics 2006; 118: e934–e963.
Antshel KM, Epstein IO, Waisbren SE : Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: a descriptive study. Neuropsychology 2004; 18: 658–664.
Bosch AM, Grootenhuis MA, Bakker HD, Heijmans HS, Wijburg FA, Last BF : Living with classical galactosemia: health-related quality of life consequences. Pediatrics 2004; 113: e423–e428.
Gitzelmann R, Steinmann B : Galactosemia: how does long-term treatment change the outcome? Enzyme 1984; 32: 37–46.
Lambert C, Boneh A : The impact of galactosaemia on quality of life—a pilot study. J Inherit Metab Dis 2004; 27: 601–608.
Ridel KR, Leslie ND, Gilbert DL : An updated review of the long-term neurological effects of galactosemia. Pediatr Neurol 2005; 33: 153–161.
Waggoner DD, Buist NRM, Donnell GN : Long-term prognosis in galactosemia: results of a survey of 350 cases. J Inherit Metab Dis 1990; 13: 802–818.
Waggoner D, Buist NRM : Long-term complications in treated galactosemia - 175 U.S. cases. Int Pediatr 1993; 8: 97–100.
Waisbren SE, Rones M, Read CY, Marsden D, Levy HL : Brief report: predictors of parenting stress among parents of children with biochemical genetic disorders. J Pediatr Psychol 2004; 29: 565–570.
Waisbren SE, Albers S, Amato S et al: Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA 2003; 290: 2564–2572.
Waisbren S, Potter N, Gordon C et al: The adult galactosemic phenotype. J Inherit Metab Dis 2012; 35: 279–286.
Douglas HC, Hawthorne DC : Enzymatic expression and genetic linkage of genes controlling galactose utilization in Saccharomyces. Genetics 1964; 49: 837–844.
Douglas HC, Hawthorne DC : Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics 1966; 54: 911–916.
Leslie ND, Yager KL, McNamara PD, Segal S : A mouse model of galactose-1-phosphate uridyl transferase deficiency. Biochem Mol Med 1996; 59: 7–12.
Ning C, Reynolds R, Chen J et al: Galactose metabolism in mice with galactose-1-phosphate uridyltransferase deficiency: sucklings and 7-week-old animals fed a high-galactose diet. Mol Genet Metab 2001; 72: 306–315.
Ning C, Reynolds R, Chen J et al: Galactose metabolism by the mouse with galactose-1-phosphate uridyltransferase deficiency. Pediatr Res 2000; 48: 211–217.
Kushner RF, Ryan EL, Sefton JM et al: A Drosophila melanogaster model of classic galactosemia. Dis Model Mech 2010; 3: 618–627.
Li Y, Ptolemy AS, Harmonay L, Kellogg M, Berry GT : Ultra fast and sensitive liquid chromatography tandem mass spectrometry based assay for galactose-1-phosphate uridylyltransferase and galactokinase deficiencies. Mol Genet Metab 2011; 102: 33–40.
Sies H, Akerboom TP : Glutathione disulfide (GSSG) efflux from cells and tissues. Methods Enzymol 1984; 105: 445–451.
Ragueneau S : Early development in mice. IV: quantity and gross composition of milk in five inbred strains. Physiol Behav 1987; 40: 431–435.
Bouwman H, Becker PJ, Cooppan RM, Reinecke AJ : Transfer of DDT used in malaria control to infants via breast milk. Bull World Health Organ 1992; 70: 241–250.
Bouwman H, Reinecke AJ, Cooppan RM, Becker PJ : Factors affecting levels of DDT and metabolites in human breast milk from Kwazulu. J Toxicol Environ Health 1990; 31: 93–115.
Prelusky DB, Veira DM, Trenholm HL, Foster BC : Metabolic fate and elimination in milk, urine and bile of deoxynivalenol following administration to lactating sheep. J Environ Sci Health B 1987; 22: 125–148.
Drugs. AAoPCo: Transfer of drugs and other chemicals into human milk. Pediatrics 2001; 108: 776–789.
Wilson JT : Transfer of drugs and other chemicals into human milk. Pediatrics 1990; 86: 149–150.
Jumbo-Lucioni PP, Hopson ML, Hang D, Liang Y, Jones DP, Fridovich-Keil JL : Oxidative stress contributes to outcome severity in a Drosophila melanogaster model of classic galactosemia. Dis Model Mech 2013; 6: 84–94.
Lai K, Yin X, Tang M, Baffoe S, Johnson B, Bodamer O : Galactose-induced lethality and growth retardation in a new galactose-1-phosphate uridyltransferase (GALT) gene-knockout mouse model; in: McCabe ERB (ed): Annual Meeting for Society for Inherited Metabolic Disorders. Elsevier: Charlotte, NC, 2012, p 330.
Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G : Definition and prevalence of subfertility and infertility. Hum Reprod 2005; 20: 1144–1147.
Ross KL, Davis CN, Fridovich-Keil JL : Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol Genet Metab 2004; 83: 103–116.
Slepak T, Tang M, Addo F, Lai K : Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Mol Genet Metab 2005; 86: 360–371.
Lai KW, Cheng LY, Cheung AL, WS O : Inhibitor of apoptosis proteins and ovarian dysfunction in galactosemic rats. Cell Tissue Res 2003; 311: 417–425.
Liu G, Shi F, Blas-Machado U et al: Dietary galactose inhibits GDF-9 mediated follicular development in the rat ovary. Reprod Toxicol 2006; 21: 26–33.
Halperin J, Devi SY, Elizur S et al: Prolactin signaling through the short form of its receptor represses forkhead transcription factor FOXO3 and its target gene galt causing a severe ovarian defect. Mol Endocrinol 2008; 22: 513–522.
Levy HL, Sepe SJ, Shih VE, Vawter GF, Klein JO : Sepsis due to Escherichia coli in neonates with galactosemia. N Engl J Med 1977; 297: 823–825.
Kelly S : Septicemia in galactosemia. JAMA 1971; 216: 330.
Takci S, Kadayifcilar S, Coskun T, Yigit S, Hismi B : A rare galactosemia complication: vitreous hemorrhage. JIMD Rep 2012; 5: 89–93.
Levy HL, Brown AE, Williams SE, de Juan E Jr : Vitreous hemorrhage as an ophthalmic complication of galactosemia. J Pediatr 1996; 129: 922–925.
Raju TS, Briggs JB, Borge SM, Jones AJ : Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 2000; 10: 477–486.
Snouwaert JN, Brigman KK, Latour AM et al: An animal model for cystic fibrosis made by gene targeting. Science 1992; 257: 1083–1088.
Hodges CA, Cotton CU, Palmert MR, Drumm ML : Generation of a conditional null allele for Cftr in mice. Genesis 2008; 46: 546–552.
Acknowledgements
We thank the expertise and advice of the University of Utah Transgenic and Gene Knockout Mouse Facility for their technical help in this project. We are also in debt to Professors Nicola Longo and Mary Bronner for their critical reading of the manuscript. Grant support (to KL) include the followings: University of Utah Faculty Start-up fund, NIH grants 5R01HD054744-06 and 1R01HD074844-01, a Research Grant from the Galactosemia Foundation (USA), and a research gift from Agios Pharmaceuticals Inc. (Boston, MA, USA). This article is dedicated to Professor Louis J Elsas, II (1937–2012) for his contributions in advancing our understanding of Classic Galactosemia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Tang, M., Siddiqi, A., Witt, B. et al. Subfertility and growth restriction in a new galactose-1 phosphate uridylyltransferase (GALT) - deficient mouse model. Eur J Hum Genet 22, 1172–1179 (2014). https://doi.org/10.1038/ejhg.2014.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2014.12