Abstract
Purpose
Oxidative stress and antioxidant status were determined in forty healthy men and postmenopausal women aged 50–70 years (F25, M15), who underwent concurrent eye examinations.
Methods
Blood samples were collected for analysing major well-known antioxidants by HPLC systems with UV and ECD detectors, total antioxidant performance using a fluorometry, lipid peroxidation determined by malondialdehyde using a HPLC system with a fluorescent detector and by total hydroxyoctadecadienoic acid (HODE) and F2-isoprotanes (8-iso-PGF2α) using GC-MS.
Results
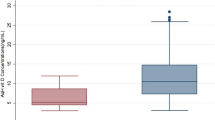
Twenty-seven (F17, M10) of the 40 subjects were diagnosed to have early cataracts at the onset of the study, which were regarded as age appropriate lens opacities. There was no significant difference in plasma major antioxidants, total antioxidant performance, and lipid peroxidation determined by malondialdehyde as well as 8-iso-PGF2α between the groups with and without early cataract. However, isomers of 9- and 13-(Z,E)-HODE levels were significantly higher in subjects with early cataract as compared with those of non-cataract subjects (P<0.05).
Conclusion
Our data suggest that subjects with early cataract are under increased systemic oxidative stress, which can be identified by a sensitive biomarker of lipid peroxidation, such as isomers of HODE.
Similar content being viewed by others
Introduction
Cataract is an opaqueness of the lens that causes decreased visual acuity.1 Cataract becomes more common with increasing age and is one of the leading causes of blindness and visual impairment among the elderly population throughout the developing world.2 Thus, identification of factors that could delay or prevent cataract development would be important both for increasing the well-being of older adults and for reducing medical care costs. Multiple factors, such as genetics, sunlight exposure, and cigarette smoking are suggested to be involved3, 4, 5, 6, 7, 8, 9 in the progression of lens opaqueness. In particular, oxidative stress is thought to have a major function in the aetiology of age-related cataract.9, 10, 11, 12, 13 Lens opaqueness is generally considered to be a common age-related progression and ages above 50 years have increased risk of developing all types of cataract.14 Therefore, oxidative stress and antioxidant status in forty men and postmenopausal women aged 50–70 years, who underwent eye examination, were determined.
Materials and methods
Subjects
Forty non-smoking men and postmenopausal women aged 50–70 years (M15, F25) were enrolled in this study. All of the study subjects were in good health, as determined by a medical history questionnaire, physical examination, and normal results of clinical laboratory tests. None of the study subjects had a history of cardiovascular, hepatic, gastrointestinal, or renal diseases, none were alcoholic, all were non-smokers and none used exogenous hormones. Subjects were not permitted to take any supplemental vitamin or carotenoid for more than 6 weeks before the start of the study and were limited to drinking less than two cups of tea per day during this 6 week period. The study protocol was approved by the Institutional Review Board of Tufts Medical Center and Tufts University Health Sciences, and written informed consent was obtained from each study subject.
Fasting (14-h) blood samples were collected in evacuated containers containing EDTA. Plasma samples were divided into aliquots and stored at −80°C for subsequent biochemical analyses. Subjects underwent eye examination at the New England Eye Center. The lens opacities were classified by a single ophthalmologist using slit lamp photographs of the nucleus and retroillumination lens photographs of both eyes and the grading system was 0=no cataract, 1=mild cataract, 2=moderate cataract, 3=severe cataract view to the fundus impaired, and 4=no red reflex.
This study protocol was designed primarily to examine the effect of antioxidants on macular pigments, but this paper focuses on antioxidant status and oxidative stress status in relation to early cataract.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers/animals were followed during this research.
Chemicals and reagents
All-trans-ß-carotene (type II), lycopene, α-tocopherol, glutathione (GSH), ascorbic acid, uric acid, human serum albumin, and soybean phosphatidylcholine were purchased from Sigma Chemical Co. (St Louis, MO, USA). Lutein was purchased from Kemin Industries (Des Moines, IA, USA). The fatty-acid analogue 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (BODIPY 581/591) was purchased from Molecular Probes (Eugene, OR, USA). The lipophilic radical initiator, 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile; MeO-AMVN), and 2,2′-azobis(2,4-dimethylvaleronitrile (AMVN) were purchased from Wako Chemicals (Richmond, VA, USA). Delipidized human serum was purchased from SeraCare Life Sciences Inc. (Oceanside, CA, USA). All HPLC solvents were obtained from JT Baker Chemical and were filtered through a 0.45-μm membrane filter before use.
Dietary assessment
Fruits and vegetable intakes were assessed with Fred Hutchinson Food Frequency Questionnaire15, 16 and calculated using Nutrition Data System for Research software version 4.04 32, developed by the Nutrition Coordinating Center, University of Minnesota, MN, USA. The questionnaires were self administered and reviewed for completeness by a research dietician.
Plasma carotenoid and tocopherol analyses
Plasma carotenoid concentrations were measured by an HPLC system as previously described with minor modification.17 Plasma samples (200 μl) were extracted with 2 ml of chloroform : methanol (2 : 1) followed by 3 ml of hexane. Samples were dried under nitrogen and resuspended in 75 μl ethanol : methyl tert-butyl ether (2 : 1) of which 25 μl was injected onto the HPLC. The HPLC system consisted of a Waters 2695 Separation Module, 2996 Photodiode Array Detector, a Waters 2475 Multi-λ Fluorescence Detector, a C30 carotenoid column (3 μm, 150 × 3.0 mm, YMC, Wilmington, NC, USA), and a Waters Millenium 32 data station. The mobile phase was methanol : methyl tert-butyl ether : water (85 : 12 : 3 by volume with 1.5% ammonium acetate in water, solvent (A) and methanol : methyl tert-butyl ether : water (8 : 90 : 2 by volume with 1% ammonium acetate in water, solvent (B). The gradient procedure has been reported earlier.17 Results were adjusted by an internal standard containing echinenone and retinyl acetate. The CV for interassay (n=25) was 4% and intraassay was 4% (n=9). Recovery of the internal standard averaged 97%.
Measurement of total antioxidant performance (TAP)
The fluorescent probe BODIPY 581/591 was incorporated into the lipid compartment of plasma at a final concentration of 2 μmol/l, as reported previously.18, 19 Samples were then diluted 1 : 10 (v/v) with PBS and incubated at 37°C with MeO-AMVN (2 mM). The lipid oxidation kinetics were monitored by the measuring the green fluorescence (λex=500, λem=520 nm) of the oxidation product of BODIPY 581/591 using a multiwell plate reader (Wallack Victor 2, PerkinElmer, Boston, USA).
Measurements of lipid peroxidation
MDA analysis
Lipid peroxidation was assessed by the measurement of malondialdehyde (MDA) using an HPLC system as reported previously.20 Briefly, plasma or plasma incubated with 5 mM of AMVN at 42°C for 2 h was treated with BHT (5% in EtOH), followed by protein precipitation using TCA (10% w/v). The mixture was reacted with TBA (0.4% w/v, in acetate buffer, pH 3.5) and analysed for MDA-TBA adducts by an HPLC system with a Pecosphere-3 C18 column (83 × 4.6 mm) using a fluorescence detector (Waters 2475 multi-λ), which was set Ex 515 nm and Em 553 nm. The HPLC mobile phase was 20 mM potassium phosphate buffe : acetonitril (80 : 20, by vol), and the flow rate was set at 0.8 ml/min. The lower limit of detection is 0.2 pmol for the MDA–TBA adduct.
Total HODE and 8-iso-PGF2α analyses
Total HODE and 8-iso-PGF2α were measured by GC/MS as reported previously21 with slight modification. Briefly, plasma was reduced with an excessive amount of sodium borohydride followed by saponification with potassium hydroxide. The identification and quantification of total hydroxyoctadecadienoic acid (HODE) and 8-iso-PGF2α were determined by their retention times and mass patterns (m/z=440, 369, 225 for HODE and 571, 481 for 8-iso-PGF2α), and ions at 440 and 481 were selected for quantification for HODE and 8-iso-PGF2α, respectively, using the internal standard 8-iso-PGF2α–d4 (m/z=485). The isomers of 9-(Z,E)- and 13-(Z,E)-HODE, 9-(E,E)-HODE, and 13-(E,E)-HODE were adequately separated using this method.
Statistical analysis
All values are presented as means±SD. Comparisons between two groups with and without early cataract were made by Student's t-test. When the normality test failed, a Mann–Whitney rank sum test was performed. Data analysis was carried out with Graph Pad Software, Prism (Version 4.0, San Diego, CA) and SigmaStat (Ver 3.1, Systat Software Inc., Point Richmond, CA, USA).
Results
Characteristics of study subjects with or without early cataract are presented in Table 1. Initial clinical laboratory test values, such as albumin, cholesterol, and triglycerides were not significantly different between the two groups and were all in normal range. Table 2 shows mean plasma concentrations of water- and fat-soluble antioxidants. No significant difference was found in major plasma antioxidants, such as carotenoids, tocopherol, ascorbic acid, and uric acid between the two groups. There was also no significant difference in total antioxidant performance between the two groups. Among various biomarkers of lipid peroxidation, such as MDA, isoprostanes (8-iso-PGF2α) and HODE, only isomers of 9-(Z,E)-HODE and 13-(Z,E)-HODE showed significantly higher values (P<0.05) in subjects with early cataracts as compared to those of subjects without any lens opaqueness (Table 3). It may still be noteworthy that, although statistically not significant, both total HODE and (E,E)-HODE observed for subjects with early cataracts were higher than those of healthy subjects.
Discussion
Epidemiological studies consistently suggest that oxidative damage is a primary event in the pathogenesis of many forms of cataracts.3, 4, 5, 6, 7, 8, 9 In addition, biochemical studies22, 23 have revealed the increased lipid peroxidation products, which can be diffused from the retina to the lens through vitreous body on degeneration of the photoreceptors,24 in human catarctous lenses.
The significantly higher plasma concentrations of (Z,E) isomers of HODE, oxidation products of linoleic acid, in subjects with early cataract in this study indicates that systemic oxidative stress could have a function in the progression of lens opaqueness. Considering linoleic acid as the most abundant circulating polyunsaturated fatty acid25, 26 in humans, HODE may give a better sensitivity and specificity detecting in vivo systemic lipid peroxidation as compared to those of other biomarkers of lipid peroxidation, such as F2-isoprostane, the oxidation products of arachidonic acid,27 or MDA, the oxidation end product of any polyunsaturated fatty acid.28 Even though the ratio of stereoisomers of 9-(Z,E)-, 13-(Z,E)-, 9-(E,E)-, and 13-(E,E)-HODE was reported to be an indicative of lipid peroxidation,19 this study suggests that the isomeric difference does not represent systemic oxidative stress. However, biological significance of total HODE as well as its isomeric configuration should be further investigated in a large study population considering the fact that the present study showed higher trend (P=0.078) of plasma total HODE concentrations in subjects with early cataract as compared to those without cataract in a rather limited study population.
Several studies have shown an inverse association between an elevated consumption of dark green vegetables, which are rich in lutein and zeaxanthin, and a decreased risk of oxidative stress-associated eye diseases, such as cataract and macular degeneration.29, 30, 31, 32, 33, 34, 35 Lutein and zeaxanthin are the only carotenoids that have been reported to be present in the lens and macula.36, 37, 38, 39Although current study showed no significant difference in fruits and vegetable intakes between subjects with and without early cataract (data are not shown), a large scale (n=35 274) of prospective study40 indicated that antioxidant rich high fruits and vegetable intakes have a modest protective effect on cataract.
Even though Knekt et al29 indicated in a case–control study that low serum concentration of β-carotene was a risk factor for end stage senile cataract, no significant difference was found in the plasma concentrations of lutein, β-carotene, lycopene, or total carotenoids in this study. It should be noted that our study subjects were all in a very early stage of cataract, whereas in Knekt et al's29 study, cataract participants had mature senile cataracts that required extraction, and that our study subjects were in a good health except for age-related progression of eye status. It is interesting to note the recent report41 conducted in a North Indian population, who were in antioxidant nutrient deficient status, showing the inverse relationship between the blood antioxidants, such as vitamin C, lutein, β-carotene and lycopene, and cataract.
Although subjects with early cataract showed significantly higher lipid peroxidation determined by (Z,E) isomers of HODE than those of subjects without cataract, plasma individual antioxidant concentrations, such as carotenoids, tocopherol, ascorbic acid, and uric acid were not differ between the two groups with and without early cataract. Therefore, it is not surprising to see no significant difference between the two groups in total antioxidant performance, which determines the ability of a biological system to quench free radicals produced by an exogenous pro-oxidant, considering that antioxidants and their interactions have an important function in total antioxidant capacity in plasma.42
In conclusion, our study clearly indicates that although overall antioxidant status was not significantly different between subjects with and without early cataract, subjects with early cataract are under systemic oxidative stress, which can be identified by a sensitive biomarker of lipid peroxidation, such as isomers of HODE.
References
Jacques PF, Chylack Jr LT, Hankinson SE, Khu PM, Rogers G, Friend J et al. Long-term Nutrient Intake and Early Age-Related Nuclear Lens Opacities. Arch Ophthalmol 2001; 119: 1009–1019.
Thylefors B, Negrel A-D, Pararajasegaram R, Dadzie KY . Global data on blindness. Bulletin of the World Health Organization 1995; 73: 115–121.
Mares-Perlman JA, Brady WE, Klein BEK, Klein R, Pelta M, Bowen P et al. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Invest Ophthalmol Vis Sci 1995; 36: 276–288.
Jacques PF, Chylack Jr LT, Taylor A . Relationships between natural antioxidants and cataract formation. In: Frei B (ed). Natural Antioxidants in Human Health and Disease. Academic Press: San Diego, 1994, pp 515–533.
Delcourt C, Cristol JP, Tessier F, Leger CL, Michel F, Papoz L . Risk factors for cortical, nuclear and posterior subcapsular cataracts: the POLA study. Am J Epidemiol 2000; 151: 497–504.
Taylor A, Jacques PF, Dorey CK . Oxidation and aging: impact on vision. Toxicol and Health 1993; 9: 349–371.
Tavani A, Negri E, La Vecchia C . Selected diseases and risk of cataract in women: a case-control study from northern Italy. Ann Epidemiol 1995; 5: 234–238.
McCarty C, Taylor HR . Light and risk for age-related eye diseases. In: Taylor A (ed). Nutritional and Environmental Influences on the Eye. CRC Press: Boca Raton, FL, 1999, pp 135–150.
Berendschot TT, Broekmans WM, Klopping-Ketelaars IA, Kardinaal AF, Van Poppel G, Van Norren D . Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch Ophthalmol 2002; 120: 1732–1737.
Jacques PF, Taylor A . Micronutrients and age-related cataracts. In: Bendich A, Butterworth CE Jr (eds). Micronutrients in health and disease prevention. Marcel Dekker: New York, 1991; 359–379.
Bhuyan KC, Bhuyan DK . Molecular mechanism of cataractogenesis: III. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Curr Eye Res 1984; 3: 67–81.
Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE et al. Nutrient intake and cataract extraction in women: A prospective study. Br Med J 1992; 305: 335–339.
Olmedilla B, Granado F, Blanco I, Vaquero M . Lutein, but not α-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition 2003; 19: 21–24.
Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA . Development of cataract and associated risk factors. Arch Ophthalmol 2006; 124: 79–85.
Kristal AR, Abrams BF, Thornquist MD, Disogra L, Croyle RT, Shattuck AL et al. Development and validation of a food use checklist for evaluation of community nutrition interventions. Am J Public Health 1990; 80: 1318–1322.
Block G, Woods M, Potosky A, Clifford C . Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990; 43: 1327–1335.
Yeum K-J, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr 1996; 64: 594–602.
Aldini G, Yeum K-J, Russell RM, Krinsky NI . A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free Radic Biol Med 2001; 31: 1043–1050.
Beretta G, Aldini G, Facino RM, Russell RM, Krinsky NI, Yeum K-Y . Total antioxidant performance: A validated fluorescence assay for the measurement of plasma oxidizability. Anal Biochem 2006; 354: 290–298.
Yeum K-J, Aldini G, Johnson EJ, Russell RM, Krinsky NI . Effect of Feeding and then depleting a high fruit and vegetable diet on oxidizability in human serum. AOCS Press: Champaign, IL, 2005.
Yoshida Y, Niki E . Bio-markers of lipid peroxidation in vivo; hydroxyoctadecaenoic acid and hydroxycholesterol. Biofactors 2006; 27: 195–202.
Micelli-Ferrari T, Vendemiale G, Grattagliano I, Boscia F, Arnese L, Altomare E et al. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br J Ophthalmol 1996; 80: 840–843.
Babizhaev MA, Shvedova AA, Arkhipenko IuV, Kagan VE . Accumulation of lipid peroxidation products in cataractous lenses. Biull Eksp Biol Med 1985; 100: 299–301.
Babizhayev MA, Deyev AI . Lens opacity induced by lipid peroxidation products as a model of cataract associated with retinal disease. Biochim Biophys Acta 1989; 1004: 124–133.
Turpeinen AM, Barlund S, Freese R, Brenna JT . Effects of conjugated linoleic acid on linoleic and linolenic acid metabolism in man. Br J Nutr 2006; 95: 727–733.
Evans JD, Waldron JM, Oleksyshyn NL . Polyunsaturated fatty acids in normal human blood. J Biol Chem 1956; 218: 255–259.
Matayatsuk C, Lee CY, Kalpravidh RW, Sirankapracha P, Wilairat P, Fucharoen S et al. Elevated F2-isoprostanes in thalassemic patients. Free Radic Biol Med 2007; 43: 1649–1655.
Peterson NC, Servinsky MD . Development of molecular cellular biomarkers of pain. Comp Med 2007; 57: 554–562.
Knekt P, Heliovaara M, Rissanen A, Aromaa A, Aaran R-K . Serum antioxidant vitamins risk of cataract. Br Med J 1992; 305: 1392–1394.
Jacques PF, Chylack Jr LT . Epidemiologic evidence of a role for the antioxidant vitamins carotenoids in cataract prevention. Am J Clin Nutr 1991; 53 (suppl): 352S–355S.
Jacques PF, Hartz SC, Chylack Jr LT, McGandy RB, Sadowski JA . Nutritional status in persons with and without senile cataracts: blood vitamin and mineral levels. Am J Clin Nutr 1988; 48: 152–158.
Mares-Perlman JA, Brady WE, Klein BE, Klein R, Palta M, Bowen P et al. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Invest Ophthalmol Vis Sci 1995; 36: 276–288.
Mares-Perlman JA, Brady WE, Klein BE, Klein R, Haus GJ, Palta M et al. Diet and nuclear lens opacities. Am J Epidemiol 1995; 141: 322–334.
Tavani A, Negri F, LaVecchia C . Food and nutrient intake and risk of cataract. Ann Epidemiol 1996; 6: 41–46.
Vitale S, West S, Hallfrisch J, Alston C, Wang F, Moorman C et al. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology 1993; 4: 195–203.
Bone RA, Landrum JT, Tarsis SL . Preliminary identification of the human macular pigment. Vision Res 1985; 25: 1531–1535.
Handelman GJ, Dratz EA, Reay CC, van Kuijk FJGM . Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci 1988; 29: 850–855.
Bone RA, Landrum JT, Fernandez L, Tarsis SL . Analysis of the macular pigment by HPLC: Retinal distribution and age study. Invest Ophthalmol Vis Sci 1988; 29: 843–849.
Yeum K-J, Taylor A, Tang G, Russell RM . Measurement of carotenoids, retinoids, and tocopherols in human lens. Invest Ophthalmol Vis Sci 1995; 36: 2756–2761.
Christen WG, Liu S, Schaumberg DA, Buring JE . Fruit and vegetable intake and the risk of cataract in women. Am J Clin Nutr 2005; 81: 1417–1422.
Dherani M, Murthy GV, Gupta SK, Young IS, Maraini G, Camparini M et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Invest Ophthalmol Vis Sci 2008; 49: 3328–3335.
Yeum K-J, Russell RM, Krinsky NI, Aldini G . Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys 2004; 430: 97–103.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research has been supported in part by NEI R03EY015674 and the US Department of Agriculture, under agreement number 1950-51000-065-08S, USA. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture
Rights and permissions
About this article
Cite this article
Li, L., Duker, J., Yoshida, Y. et al. Oxidative stress and antioxidant status in older adults with early cataract. Eye 23, 1464–1468 (2009). https://doi.org/10.1038/eye.2008.281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.281
Keywords
This article is cited by
-
Aqueous humor and serum 25-Hydroxyvitamin D levels in patients with cataracts
BMC Ophthalmology (2020)