Abstract
Aims
To determine the efficacy and safety of intravitreal ranibizumab in the treatment of choroidal neovascularization (CNV) secondary to pathologic myopia (PM).
Methods
Prospective, consecutive, non-randomized, interventional case series of 23 eyes of 23 patients with CNV secondary to PM treated with intravitreal ranibizumab as needed, after the first injection (PRN: Pro Re Nata). Patients were followed-up monthly with best-corrected visual acuity (BCVA), biomicroscopy, fluorescein angiography, and optical coherence tomography.
Results
There were 23 eyes of 23 patients, and the mean age was 51.08 (SD=17.40) years. One patient was lost during the follow-up. At the 12-month follow-up, the mean VA improved by 9.53 letters (P<0.05). In all, 69% of patients increased at least one line, and 34.7% increased three or more lines. There were no cases of moderate vision loss (>3 lines) or severe vision loss (>6 lines). Favourable outcomes were obtained in all subgroups. Patients received an average of 1.52 injections. No serious ocular complications were noted.
Conclusions
The 12-month results of this consecutive series of 23 patients suggests that a small number of injections of intravitreal ranibizumab may be safe and effective for both preventing and restoring visual loss in patients with CNV secondary to PM. Further studies to evaluate the safety and efficacy are justified.
Similar content being viewed by others
Introduction
Pathological myopia (PM) is one of the leading causes of visual disability in the world from 20–50 years of age.1 Choroidal neovascularization (CNV) is one of the most important vision-threatening complications of pathologic myopia (PM) and occurs in 5–10% of myopic patients, with a positive correlation between risk and degree of myopia. Among myopic patients with pre-existing CNV, more than 30% will develop CNV in the fellow eye within 8 years.2
Photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis Ophthalmics, Basel, Switzerland) is, since 2001, the only approved treatment for subfoveal CNV in myopic eyes, and is able to stabilize best-corrected visual acuity (BCVA) of patients with subfoveal myopic CNV. At 12-month follow-up, 86% of the verteporfin-treated patients lost fewer than 15 letters of BCVA, in comparison to 67% of the placebo-treated patients.3 However, its limitations, particularly for improving vision, are well known.
In 2005, Nguyen et al4 reported good results with the use of systemic bevacizumab (Avastin, Genentech, San Francisco, CA, USA) in two myopic patients with CNV. Yamamoto et al5 and Sakaguchi et al6 were the first to report a short series of patients with the use of intravitreal bevacizumab for the treatment of subfoveal CNV secondary to PM, showing favourable results with no adverse events. The short-term results of both reports appeared promising with improvement in mean VA from 2 to 3.5 lines.
Ranibizumab (Lucentis, Novartis, Basel, Switzerland) is a humanized antibody fragment against VEGF-A that was specifically designed for intraocular use as a smaller antibody fragment to better penetrate through the retina.
Here, we present the results of a 12-month prospective study that includes eyes with CNV secondary to PM treated with intravitreal injections of bevacizumab.
Materials and methods
A prospective, consecutive, nonrandomized, interventional case series of 23 eyes of 23 patients with CNV secondary to PM was carried out. Patients with subfoveal or juxtafoveal CNV secondary to PM were recruited to receive intravitreal injections of ranibizumab as needed, after the first injection (PRN: Pro Re Nata). PM was defined as an eye with a minimum refractive error of −5 D and retinal signs, such as lacquer cracks and peripapillary chorioretinal atrophy. CNV was confirmed with fluorescein angiography (FA) and/or optical coherence tomography (OCT). Patients could have received earlier treatments for CNV before being recruited for the study.
Patients were excluded from the study if they presented features of any condition other than PM associated with CNV that could suggest a different aetiology, such as age-related macular degeneration, angioid streaks, trauma, choroiditis, or hereditary diseases in the study or the fellow eye.
All patients underwent a complete ophthalmological examination at the time of inclusion in the study, including BCVA with ETDRS charts, biomicroscopic evaluation of the anterior and posterior segment, FA, and OCT. Patients with active CNV secondary to PM were offered treatment with intravitreal ranibizumab in an ‘off-label’ fashion. The potential benefits and side effects were discussed with patients and relatives. Patients willing to receive treatment provided written informed consent.
The need for treatment was based on the presence of recurrent intraretinal or subretinal fluid on OCT scans or leakage on FA. BCVA and presence of metamorphopsia were also used to decide the need for treatment when OCT scans or FA were questionable.
The procedure was carried out under sterile conditions as an office procedure. Patients were patched for 4 h and instructed to apply ciprofloxacin 0.03% (Exocin, Allergan) one drop, five times daily for 5 days after the procedure and fluormetolone 0.25% (FML forte, Allergan) one drop, five times daily for 2 days after the procedure. Patients were contacted by telephone on the following day and instructed to come back for a consultation if symptoms of pain or significant loss of vision occurred.
Follow-up examinations were carried out every month. At each visit, another complete ophthalmic examination was carried out, including BCVA testing with ETDRS charts and fourier domain OCT scanning (3D OCT Topcon, Topcon, Japan).
The need for retreatment was based on the presence of recurrent intraretinal or subretinal fluid on OCT scans or leakage on FA. BCVA and presence or worsening of subjective metamorphopsia was also used to decide the need for retreatment when OCT scans or FA were questionable. The main outcome measures were mean change in BCVA. Secondary outcomes were angiographic cessation of leakage, and absence of intrarretinal or subretinal fluid.
Data were analysed using the paired t-test. A P-value of 0.05 was considered significant.
Results
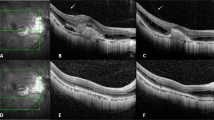
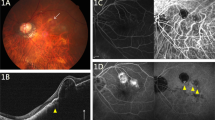
Table 1 shows the patient characteristics. In all, 23 eyes of 23 consecutive patients with CNV secondary to PM were included. Patients were enrolled between January 2007 and January 2008. Seventeen were females, and 6 were males. Mean age was 51.08 years (SD=17.40). Twelve patients were older than 50 years. The mean spherical equivalent refractive error of the 23 eyes was −10.39 D (SD=10.39). Fourteen patients were treated earlier and 9 were naïve. Six eyes were treated earlier with PDT and intravitreal bevacizumab, four eyes with intravitreal bevacizumab alone, and four eyes with PDT alone. One patient was lost during the follow-up, at month 8 (patient no. 15). The patient was contacted by telephone after 4 months, and she had not received additional injections, without, at least, any worsening of her vision. Figure 1 shows the results in a 74-year-old woman with CNV secondary to PM that had been treated earlier with 2 intravitreal injections of bevacizumab.
Patient no. 6: A 74-year-old woman who had been treated with two intravitreal injections of bevacizumab recently—4 months ago. She had very active recurrence of CNV and was treated with intravitreal ranibizumab at the onset and at 1 month as leakage was still present at FA. Leakage resolved and vision improved four lines of ETDRS.
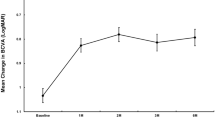
Table 2 shows the mean change in VA. At the 12-month follow-up, the mean VA improved by +9.53 letters (P<0.05). The VA improvement was higher in the untreated eyes (+12.78 letters; P<0.05) than in the earlier treated eyes with PDT and/or intravitreal bevacizumab (+7.43 letters; P<0.05). Patients with an age <50 years improved by a mean of +12 letters (P<0.05), and patients older than 50 years improved by a mean of +7.25 letters (P<0.05). Overall, 16 cases (69.5%) increased at least one line of VA (Table 3). Six eyes (27%) increased more than three lines of VA. There were no cases of moderate vision loss (>3 lines) or severe vision loss (>6 lines).
The mean number of injections was 1.52. Six patients (26%) were retreated during the follow-up. One eye required five injections (patient no. 1), three eyes required three injections (patients no. 13, 19, and 21), and two eyes required two injections (patients no. 6 and 16). Four of them were older than 50 years, and three were treated earlier.
No ocular complications were noted after intravitreal injections, besides one patient with partial corneal desepithelization. One patient suffered angina 2 months after a single injection, although it seemed related to a marked anaemia secondary to a hip surgery.
Discussion
Angiogenesis is regulated by several proangiogenic and antiangiogenic factors, many of which have now been identified. VEGF has been found to be one of the key elements in angiogenesis. Tong et al7 showed that the VEGF concentrations in aqueous humour were markedly increased in patients with CNV secondary to PM when compared with the controls.
Several authors recently reported the benefit of intravitreal bevacizumab for myopic CNV. Yamamoto et al5 reported a retrospective case series of 11 eyes from 9 patients injected with intravitreal bevacizumab. Bevacizumab led to both VA and anatomical improvements. These short-term results were very promising and average visual improvement was 3.5 lines of gain, and 8 of 11 eyes achieved 20/50 or better vision. Sakaguchi et al6 reported a prospective case series of eight eyes from eight patients. Six patients (75%) had an improved BCVA of two or more lines; in two (25%) patients, the BCVA remained the same. No ocular or systemic adverse events were reported.
In the present prospective, consecutive, nonrandomized, interventional case series, we report the real-life clinical experience with ranibizumab for CNV secondary to PM in 23 eyes of 23 patients. At the 12-month follow-up, the mean VA improved by 9.53 letters (P<0.05). A total of 77% of patients increased at least one line, and 4.5% increased more than six lines. There were no cases of moderate vision loss (>3 lines) or severe vision loss (>6 lines). Favourable outcomes were obtained in all subgroups. Patients received an average of 1.52 injections. No serious ocular complications were noted. The overall population and the smaller subgroups showed statisctical significance.
Both untreated and previously treated eyes showed marked VA improvement at the end of follow-up. However, the benefit was more important in the untreated eyes. Naïve lessions may have less RPE and photoreceptors damage and an increased recovery potential.
The mean number of injections at 12 months was 1.52. Some patients showed a progressive improvement beyond 1 month after a single injection. These results suggest that at least in myopic CNV, PRN treatment from the first injection may achieve good clinical results, minimizing the number of needed injections and their potential related systemic and local complications.
Although the results of this consecutive series of 23 patients and earlier series of bevacizumab for myopic CNV do not show specific risks for myopic eyes, these eyes are at a potentially increased risk of suffering retinal tears and retinal detachment. Myopic eyes might also be at an increased risk of spontaneous choriorretinal atrophy after receiving treatments, such as laser or PDT. So far, we have not found any evidence of increased incidence of retinal pigment epithelium, choriocapillaris damage, and atrophy in this series of 23 patients. However, this could change with a longer follow-up. Moreover, paninhibition of VEGF might impact in the survival of retinal neurons,8 so it is desirable to administer the smallest number of injections possible, especially in myopic eyes.
A 77-year-old male patient suffered three angina pectoris 2 months after his first single injection. It seemed to be related to a marked anaemia secondary to a hip surgery.
The limitations of this study include its small number of eyes, the relatively short follow-up, and the absence of a control group. The number of patients is too small for further subgroup analysis of subtype lesion. However, all types of lesions showed a dramatic response to treatment, requiring very few injections. One patient was lost during the follow-up, at the 8th month (patient no. 15). As the series is small, a single patient with an unfavourable outcome might have driven the results to a different level of efficacy. The patient was contacted by telephone after 4 months, and she had not received additional injections, without, at least, any worsening of her vision. Therefore, this dropout does not appear to bias our favourable results.
In summary, the results are promising, with a small number of injections needed to preserve vision, and a large proportion of patients with marked gain. Longer follow-up with a larger number of patients is needed to further determine the safety and efficacy of intravitreal ranibizumab in the treatment of myopic CNV.
References
Ghafour IM, Allan D, Foulds WS . Common causes of blindness and visual handicap in the west of Scotland. Br J Ophthalmol 1983; 67: 209–213.
Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL . Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 1984; 91: 1573–1581.
Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial—VIP report no. 1. Ophthalmology 2001; 108: 841–852.
Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA . Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 2005; 89: 1368–1370.
Yamamoto I, Rogers HA, Reichel E, Yates P, Ducker J Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathologic myopia. Br J Ophthalmol 2007; 91: 157–160.
Sakaguchi H, Ikuno Y, Gomi F, Kamei M, Sawa M, Tsujikawa M et al. Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol 2007; 91: 161–165.
Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 2006; 141: 456–462.
Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptative response to ischemic injury. Am J Pathol 2007; 171 (1): 53–67.
Author information
Authors and Affiliations
Corresponding author
Additional information
Some of the content of this paper was presented at the ISOPT Meeting, Budapest February 2008
Proprietary interest: None.
Rights and permissions
About this article
Cite this article
Monés, J., Amselem, L., Serrano, A. et al. Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye 23, 1275–1281 (2009). https://doi.org/10.1038/eye.2009.88
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.88
Keywords
This article is cited by
-
Comparison of anatomical and functional outcomes of treating myopic choroidal neovascularization with bevacizumab or ranibizumab
International Ophthalmology (2023)
-
The efficacy of different anti-vascular endothelial growth factor agents and prognostic biomarkers in monitoring of the treatment for myopic choroidal neovascularization
International Ophthalmology (2022)
-
Baseline characteristics of myopic choroidal neovascularization in patients above 50 years old and prognostic factors after intravitreal conbercept treatment
Scientific Reports (2021)
-
Long-term outcomes of the intravitreal injection of ranibizumab for the treatment of choroidal neovascularization secondary to pathologic myopia
International Ophthalmology (2020)
-
Myopic Choroidal Neovascularization: Diagnosis and Treatment Update
Current Ophthalmology Reports (2019)