Abstract
Purpose
To describe the efficacy of intravitreal aflibercept on 12-month visual and anatomical outcomes in patients with neovascular age-related macular degeneration (AMD) recalcitrant to prior monthly intravitreal bevacizumab or ranibizumab.
Methods
Non-comparative case series of 21 eyes of 21 AMD patients with evidence of persistent exudation (intraretinal fluid/cysts, or subretinal fluid (SRF), or both) on spectral domain OCT despite ≥6 prior intravitreal 0.5 mg ranibizumab or 1.25 mg bevacizumab (mean 29.8±17.1 injections) over 31.6±17.4 months who were transitioned to aflibercept.
Results
At baseline, best-corrected visual acuity (BCVA) was 0.42±0.28 logarithm of minimum-angle of resolution (logMAR), central foveal thickness (CFT) was 329.38±102.67 μm and macular volume (MV) was 7.71±1.32 mm3. After 12 months of aflibercept (mean 10.2±1.2 injections), BCVA was 0.40±0.28 logMAR (P=0.5), CFT decreased to 292.71±91.35 μm (P=0.038) and MV improved to 7.33±1.27 mm3 (P=0.003). In a subset of 15 eyes with a persistent fibrovascular or serous pigment epithelial detachment (PED), mean baseline PED greatest basal diameter (GBD) was 2350.9±1067.6 μm and mean maximal height (MH) was 288.7±175.9 μm. At 12 months, GBD improved to 1896.3±782.3 μm (P=0.028), while MH decreased to 248.27±146.2 μm (P=0.002).
Conclusion
In patients with recalcitrant AMD, aflibercept led to anatomic improvement at 12 months, reduction in proportion of eyes with SRF and reduction in PED, while preserving visual acuity.
Similar content being viewed by others

Introduction
Some eyes with neovascular age-related macular degeneration (AMD) have persistent disease activity despite monthly therapy.1 Various strategies have been attempted in eyes including increasing the frequency of dosing2 by alternating treatment between ranibizumab and bevacizumab or increasing the dose of the drug while maintaining a monthly regimen.2
Efforts have also focused on switching these eyes to aflibercept.3, 4, 5, 6
In this case series review, we analyze the visual and anatomical changes following 12 months of intravitreal aflibercept in previously treated patients with recalcitrant exudative-AMD.
Case series
This was an interventional, IRB approved, non-comparative case series of consecutive patients with neovascular AMD that were switched from treatment with intravitreal ranibizumab or bevacizumab to intravitreal aflibercept for recalcitrant exudative-AMD defined as choroidal neovascularization secondary to AMD with spectral domain OCT (SD-OCT; Heidelberg Spectralis HRA+OCT, Vista, CA, USA) documentation of subretinal fluid (SRF) and/or intraretinal fluid (IRF) and/or subretinal pigment epithelium (RPE) fluid with adjacent SRF/IRF following >6 months of monthly anti-VEGF treatment. Idiopathic polypoidal choroidal vasculopathy and central serous retinopathy cases were excluded. We excluded eyes with anti-VEGF therapy <28 days prior, photodynamic therapy (PDT) <3 months prior or >4 prior PDT treatments, significant subfoveal-fibrosis (>50% of lesion), prior triamcinolone (<6 months), intraocular surgery (<2 months), history of vitrectomy, active intraocular-inflammation, vitreous hemorrhage, subretinal hemorrhage involving >1 disc area of central fovea, previous RPE tear, or best-corrected visual acuity (BCVA) <20/400.
Each subject served as his/her own historical control and was treated by the same physician using identical retreatment criteria (before/after conversion).
Twenty-one eyes of 21 patients (9 males, 12 females; mean-age 80.7±4.5 years) who had received 29.8±17.1 (range 6–70) prior ranibizumab or bevacizumab injections over 31.6±17.4 (range 7–63) months were included. Average previous injections were 11.33/year (ranibizumab) and 11.1/year (bevacizumab). Nine patients were former smokers, while 12 had never smoked and none had prior myocardial infarction or cardiovascular accident. Five eyes (24%) had received ranibizumab exclusively (mean, 27.4; range, 6–43) of which one patient had received a single prior PDT treatment, four eyes (19%) had received bevacizumab exclusively (mean, 20.4; range 6–37), and 12 eyes (57%) had been treated with both agents (mean, 33.8; range, 7–70 injections). Eyes had been treated with bevacizumab or ranibizumab monthly till signs of exudation were present. None of the eyes had vitreomacular traction or an epiretinal-membrane on baseline SD-OCT.
Patients received a minimum of three monthly aflibercept injections following which they switched to a bimonthly-interval if there was resolution of SRF/IRF, else were maintained on a monthly regimen. The interval frequency was guided by OCT.
At baseline, BCVA was 0.42±0.28 logarithm of minimum-angle of resolution (logMAR) (20/52 snellen; range 0.10–0.90 logMAR), central foveal thickness (CFT) was 329.38±102.67 μm (range 216–637 μm) and macular volume (MV) was 7.71±1.32 mm3 (range 4.83–10.15 mm3).
After 12 months of aflibercept (mean 10.2±1.2 injections; range 10–12), BCVA was 0.40±0.28 logMAR (20/50 snellen; range 0–1, P=0.5, Figure 1a), CFT decreased to 292.71±91.35 μm (range 193–627, P=0.038, Figure 1b), and MV improved to 7.33±1.27 mm3 (range 5.07–9.36, P=0.003, Figure 1c; Table 1A). There was a reduction in proportion of eyes with persistent SRF (17/21 to 10/21, P=0.05) and IRF (10/21 to 5/21 eyes, P=0.2). Figures 2 and 3 are representative case examples of the anatomical response after switching to aflibercept.
(a) Scatterplot showing a 12-month follow-up versus baseline logarithm of the minimal angle of resolution (logMAR) visual acuity after transitioning to aflibercept injections to treat recalcitrant neovascular age-related macular degeneration. (b) Scatterplot showing 12-month follow-up versus baseline central foveal thickness on SD-OCT (microns) after transitioning to aflibercept injections to treat recalcitrant neovascular age-related macular degeneration. (c) Scatterplot showing a 12-month follow-up versus baseline macular volume on SD-OCT (mm3) after transitioning to aflibercept injections to treat recalcitrant neovascular age-related macular degeneration.
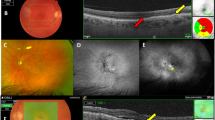
A patient with persistent SRF despite 22 prior anti-VEGF injections (1 Bevacizumab and 21 Ranibizumab) over the prior 22 months and was transitioned to aflibercept. Following 12 monthly aflibercept injections, SRF substantially reduced on SD-OCT. CFT improved from 341 to 319 μm and macular volume reduced from 10.01 to 9.34 mm3. Visual acuity was preserved at 20/60.
A patient with a persistent pigment epithelial detachment (PED) and SRF despite 45 prior ranibizumab injections over the prior 50 months, was transitioned to aflibercept. Following 12 monthly aflibercept injections, there was resolution of the PED and SRF on SD-OCT. CFT improved from 419 to 207 μm and macular volume reduced from 8.92 to 8.94 mm3. Visual acuity was preserved at 20/60.
We analyzed a subgroup of 15 eyes with a persistent fibrovascular or serous pigment epithelial detachment (PED). Using software calipers, we calculated a significant reduction of greatest basal diameter and maximal height both at 6 and 12 months (Table 1B). None of the eyes in our study developed significant ocular or systemic adverse events.
Discussion
Short-term efficacy of transitioning to aflibercept in such recalcitrant eyes has been reported.4, 5, 6, 7, 8, 9 Potential explanations for the overall anatomic success of aflibercept therapy in recalcitrant eyes include tachyphylaxis, pharmacogenetics, or the increased binding affinity of aflibercept.
The anatomic gains in our study did not translate into improved visual acuity. This is not surprising, as our patients had received a mean of 29.8 prior injections with persistent exudation. Prior studies have shown that in cases of chronic AMD, conversion from bevacizumab to ranibizumab yielded improvements in anatomy, but not visual acuity.10
We did not analyze the characteristics of eyes that failed to respond to aflibercept. It is also not yet clear whether similar but earlier intervention with aflibercept could avoid visual acuity loss and whether cases initially unresponsive to aflibercept might show similar responses when switching to another anti-VEGF medication such as ranibizumab. Most patients required monthly aflibercept, more frequent than the bimonthly dosing it is designed for as these were recalcitrant eyes. It is also possible that as we included eyes that had been followed for 12 months on aflibercept, these were cases more responsive, as non-responders may have been switched to another regimen or might elect to abandon treatment.
In conclusion, this study provides 1-year results following transition to aflibercept for previously treated eyes. We observed a significant reduction in CFT, MV, PED height, and diameter with a near 50% decrease in the number of eyes with persistent SRF/IRF.

References
Group CR, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908.
Stewart MW, Rosenfeld PJ, Penha FM, Wang F, Yehoshua Z, Bueno-Lopez E et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina 2012; 32: 434–457.
Ho VY, Yeh S, Olsen TW, Bergstrom CS, Yan J, Cribbs BE et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol 2013; 156 (1): 23–28.e2.
Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 2013; 156: 15–22. e11.
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JT, Sobrin L, Eliott D et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 2013; 156 (1): 29–35.e2.
Cho H, Shah CP, Weber M, Heier JS . Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 2013; 97: 1032–1035.
Patel KH, Chow CC, Rathod R, Mieler WF, Lim JI, Ulanski LJ 2nd et al. Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye (Lond) 2013; 27: 663–668.
Kumar N, Marsiglia M, Mrejen S, Fung A. T, Slakter J, Sorenson J et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina 2013; 33: 1605–1612.
Chaikitmongkol V, Bressler NM . Dramatic resolution of choroidal neovascular abnormalities after single aflibercept injection following years of ranibizumab use. JAMA Ophthalmol 2013; 131: 260–262.
Ehlers JP, Spirn MJ, Shah CP, Fenton GL, Baker PS, Regillo CD et al. Ranibizumab for exudative age-related macular degeneration in eyes previously treated with alternative vascular endothelial growth factor inhibitors. Ophthalmic Surg Lasers Imaging 2010; 41: 182–189.
Acknowledgements
This work was supported in part by an unrestricted grant from Research to Prevent Blindness, NY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting in May 2013 and the American Society of Retina Specialists Annual Meeting in August 2013
Rights and permissions
About this article
Cite this article
Grewal, D., Gill, M., Sarezky, D. et al. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye 28, 895–899 (2014). https://doi.org/10.1038/eye.2014.101
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.101
This article is cited by
-
Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab
International Journal of Retina and Vitreous (2021)
-
How Successful is Switching from Bevacizumab or Ranibizumab to Aflibercept in Age-Related Macular Degeneration? A Systematic Overview
Advances in Therapy (2019)
-
Clinical response of pigment epithelial detachment associated with neovascular age-related macular degeneration in switching treatment from Ranibizumab to Aflibercept
BMC Ophthalmology (2018)
-
“What should I inject next?” Challenging treatment decisions in the multiple anti-VEGF: a review of publications exploring anti-VEGF switching for nAMD
International Ophthalmology (2018)
-
Aflibercept as a Second Line Therapy for Neovascular Age Related Macular Degeneration in Israel (ASLI) study
Eye (2017)