Abstract
Purpose
The purpose of this study was to report the 2-year outcome of an individually tailored ‘observe-and-plan’ treatment regimen for neovascular age-related macular degeneration (nAMD), and to investigate its clinical value in terms of functional outcome. This regimen aimed to reduce the clinical burden (visits) by employing individually fixed injection intervals, based on the predictability of an individual’s need for retreatment.
Methods
This prospective case series included 104 patients (115 eyes) with nAMD. Following three loading doses of ranibizumab, the disease recurrence interval was determined in monthly observation visits. Retreatment was applied in a series of three injections with individually fixed intervals (2 weeks shorter than the recurrence interval), combined with periodic adjustment of the intervals. The allowed injection intervals in treatment plans ranged from 1 to 3 months. If there was no recurrence at 3 months, the patient could change to monitoring alone.
Results
Mean visual acuity (VA) improved by 8.7, 9.7, and 9.2 letters at months 3, 12, and 24, respectively. The mean number of injections was 7.8 and 5.8 during years 1 and 2, respectively, whereas the mean number of ophthalmic examinations was 4.0 and 2.9, respectively. The mean treatment interval (after the loading doses) was 2.0 months during year 1, and 2.2 months during year 2.
Conclusion
The observe-and-plan regimen significantly improved and maintained VA over the course of 2 years. This favourable functional outcome was achieved with fewer clinic visits compared with other regimens. Therefore, this observe-and-plan regimen has the potential to alleviate the clinical burden of nAMD treatment.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a frequent macular pathology. Its natural course was once the main cause of irreversible vision loss in individuals aged ≥50 years in industrialized countries.1 Since the introduction of intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment (first ranibizumab2, 3, 4 and later aflibercept5, 6) as a new gold standard for the neovascular form of AMD (nAMD), the proportion of legally blind eyes has significantly decreased.7 However, because monthly retreatment2, 3, 4 places a heavy burden on the health-care system and on patients,5 alternative treatment regimens have been explored. Simply reducing the intravitreal injections to a fixed retreatment every 3 months was significantly inferior to monthly injections and resulted in the loss of initial visual acuity (VA) improvement.8, 9, 10 Although the individually adjusted pro re nata (PRN) retreatment regimen was able to reduce the number of retreatments with (near) noninferiority of visual results as compared with monthly retreatment,11, 12 this regimen still requires monthly monitoring visits to detect disease recurrence and determine the need for retreatment. In a context of chronic care management and indefinite treatment duration, monthly monitoring visits place a heavy burden on ophthalmic institutions, with new patients being regularly added because of the high incidence of nAMD.13
We recently reported the 1-year results of an ‘observe-and-plan’ retreatment regimen designed to alleviate the clinical burden of nAMD.14 Based on the previously reported predictability of the need for retreatment,15 we developed this retreatment algorithm to allow us to predict and apply the number of retreatments that were individually adequate while reducing the number of assessment visits. VA outcome served as validation of the regimen. The first-year results of this study showed good visual results at 12 months, in combination with fewer assessment visits. We now report the results after 2 years of continuous treatment with the ‘observe-and-plan’ regimen.
Materials and methods
This prospective study was undertaken in the medical retina department of a single tertiary referral centre (University Eye Hospital Jules Gonin in Lausanne, Switzerland). The study was approved by the local ethics committee and adhered to the tenets of the Declaration of Helsinki. All patients gave written informed consent. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Patient selection
Details about the patient selection were described in our previous report of the 1-year results.14 In summary, inclusion criteria were treatment-naive nAMD with active subfoveal choroidal neovascularization (CNV), best corrected visual acuity (BCVA) from 20/25 to 20/400, a maximum lesion size of 12 disc areas, and informed consent. Patients presenting with subfoveal atrophy or fibrosis in the centre of the macula were excluded, as well as those with any confounding other macular pathology, or the inability to obtain retinal imaging of sufficient quality.
Clinical investigations
Baseline examination and all subsequent follow-up visits included measurement of BCVA on the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, slit-lamp examination, measurement of intraocular pressure (IOP), dilated fundus examination, and spectral domain OCT (SD-OCT; Cirrus, Carl Zeiss Meditec, Inc., Oberkochen, Germany). The following additional examinations were performed at baseline and after 3 months using the Topcon TRC-50IX (Tokyo, Japan): fundus autofluorescence imaging, fluorescein angiography, and indocyanine green angiography (the latter at baseline only).
OCT analysis
A macular cube (512 × 126) scan captured using an SD-OCT Cirrus machine (Carl Zeiss Meditec, Inc.) was used for baseline examination and all follow-up visits. The macular thickness map was acquired with the Integrated Software 6.0.0.599 that centred on the automatically identified foveal pit and was manually corrected if needed. Central retinal thickness (CRT) was measured in the central 1 mm2 subfield. All OCT scans were qualitatively evaluated for the presence or absence of intra- or subretinal fluid.
Observe-and-plan regimen
The methodology of the ‘observe-and-plan’ regimen has been described previously in detail.14 The key concept of the observe-and-plan regimen was to evaluate the individual need for retreatment (after three loading doses), and then apply the optimal interval in a fixed treatment plan of a series of injections. The interval was regularly adjusted for the subsequent treatment plans. A flowchart diagram of the regimen is available in the previous publication regarding the 1-year results.14
In detail, three loading doses of 0.5 mg ranibizumab (Lucentis, Novartis Pharma AG, Basel, Switzerland) were followed by monthly evaluation visits with complete ophthalmic examination as described above. (In case of recurrence-free macula, the monthly rhythm of these visits could be extended to 1.5 months after 3 months, and to 2 months after 6 months since last injection.) When on examination exudative activity appeared on SD-OCT or fundus examination, the interval from last injection was shortened to the next shorter available treatment interval ranging from 1 to 3 months. This interval was applied for 2–3 injections (treatment plan), followed by an assessment visit after 3–6 months since last assessment. The available choices of treatment plans were: 3 injections at 1-month, 1.5-month, or 2-month intervals; or 2 injections at 2.5-month or 3-month intervals. At the assessment visits, the next treatment plan was adjusted by one step: the next shorter interval if exudative signs were detected; otherwise the next longer interval. If the macula was still dry after a 3-month interval plan, the eye was monitored every 1.5 months.
Per protocol, a variation of ±1 week was accepted for visits and injections. However, some patients missed their appointments for various reasons and needed to be rescheduled. A delay of up to 3 weeks was accepted without dropout of the study.
Clinical outcome analysis
The main outcome for clinical validation of the ‘observe-and-plan’ regimen included: the mean BCVA change over time with an end point at 12 and 24 months, the proportion of eyes that lost >15 letters, and the proportion of eyes that gained ≥15 letters. Additional parameters were mean CRT change, the treatment intervals over time, the number of visits and injections over 2 years, and the presence of fluid.
An economic assessment of this regimen in comparison with other variable treatment protocols was completed (See Supplementary Information).
Statistical evaluation
Patients were not seen for monthly visits on this treatment plan, and hence the last available BCVA and CRT measurements were carried forward to the last visit to allow for statistical analysis. However, missing data for patients that were lost to follow-up were taken into account until their last visit only.
Apart from descriptive statistics, a paired t-test was used to compare visual acuity at different time points. We also used the McNemar test to analyse the individual interval category during year 1 vs year 2. Categorical distribution was analysed with χ2 test. Statistical results were considered significant at a level of significance of 0.05.
Results
Study population
Baseline characteristics were described in detail in our report of the 1-year results.14 In summary, 115 eyes from 104 Caucasian patients (mean age, 79.5 years; 63.5% women) were included. Mean baseline BCVA was 58.3 ETDRS letters (Snellen equivalent 20/80+3, SD 18.0).
Two patients (2 eyes) were lost during the first year, and 7 patients (8 eyes) were lost to follow-up during the second year.
Improvements in VA
Mean BCVA improved by 8.7 letters (P<0.0001, paired t-test) at month 3 and this increase was maintained at months 12 and 24 (+9.7 and +9.2 letters, respectively) (Figure 1; upper graph). The proportion of eyes gaining ≥15 letters, gaining ≥0 letters, and losing <15 letters were 30%, 83%, and 97%, respectively, after 12 months, and 33%, 80%, and 96%, respectively, after 24 months.
Mean change of best corrected visual acuity (BCVA; upper graph) and of central retinal thickness (CRT; lower graph) measured using optical coherence tomography of all study eyes treated with ranibizumab for neovascular age-related macular degeneration following an observe-and-plan regimen during the 24-month study period. Error bars represent SEM.
Factors affecting clinical burden
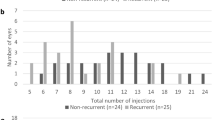
The mean number of clinic visits with ophthalmic examinations after baseline was 6.9 (SD 2.3) that divided into 4.0 (SD 1.4) and 2.9 (SD 1.2) during the first and the second year, respectively. The distribution of number of visits and injections over the 2-year course of the study is shown in Figure 2.
The mean number of injections was 13.6 (SD 6.1, range 3–25), including the first three loading doses. This divided into 7.8 (SD 3.1, including three loading doses) and 5.8 (SD 3.4) in the first and second year, respectively. The mean treatment interval after the loading doses was 2.0 months (SD 3.1) in the first year and 2.2 months (SD 3.3) in the second year that was not significantly different (P=0.835).
Subgroups were analysed according to the treatment interval (individual average) during the first and the second year of follow-up, respectively: a short retreatment interval of up to 1.5 months, an intermediate interval longer than 1.5 but shorter than 3 months, or a long retreatment interval of ≥3 months. The comparison revealed that 67.3% of patients remained in the same category from year 1 to year 2, whereas 32.7% changed their category form year 1 to year 2 (Table 1). Short intervals in the first year (≤1.5 months) had a 46.3% chance of extending their interval beyond 1.5 months during the second year. Overall, no significant change was found (P=0.074).
In addition, the very first measured interval (after the loading doses) and the last interval at month 24 were compared. These values were plotted against each other to show the variability of evolution observed (Figure 3). Inherent to the regimen, the treatment interval changes by one step forward and backward, even in the case of perfectly regular recurrences. Therefore, change by one step was considered a stable interval, whereas changes by two steps or more were defined as longer/shorter. We found that 60 eyes were stable (57.1%) between their first and last interval, 33 eyes (31.4%) showed longer intervals (less treatment need) over time, and 12 eyes (11.4%) needed shorter intervals (higher treatment need).
Distribution of the first measured interval after loading doses (horizontal axis), plotted against the last applied interval at month 24 (vertical axis), for all eyes that underwent treatment with ranibizumab for neovascular age-related macular degeneration according to the studied regimen ‘observe and plan’. The term ‘observation’ is equivalent to any interval longer than 3 months. These eyes were followed regularly without planned injection.
Ten patients had both eyes included into the study and completed the 24 months. Four of them showed very similar need of retreatment in both eyes, with 0–20% difference between the treatment interval means. However, the paired comparison of the treatment interval means over 2 years for all 10 patients showed a statistical trend toward a difference between the 2 eyes, with a P-value of 0.063 (Wilcoxon signed-rank test).
Structural outcome
OCT measurements demonstrated that mean CRT improved from 342 μm (SD 85) at baseline to a mean of −97, −99, and −96 μm at months 3, 12, and 24, respectively (Figure 1; lower graph). The proportion of eyes with presence of fluid (intraretinal or subretinal) on SD-OCT was analysed for the last available visit before month 12 and month 24. This proportion was 46% and 43.8%, respectively. The distribution between the groups with short, intermediate, and long intervals was not significantly different for the proportion of eyes with fluid at 1 year (χ2 test 0.11), but showed a significantly higher proportion in the short intervals at 2 years (χ2 test <0.001; Table 2).
Safety
No severe ocular or systemic adverse events were reported during the course of this study. In two instances, the investigators decided to apply the protocol option to shorten the interval more than normally suggested. The reason for this was that recurrence was considered more severe than expected. Both events happened during the second year of ‘observe-and-plan’, and both occurred after a treatment plan of two injections at 3-month intervals that was preceded by a prolonged phase of monitoring without signs of exudation and without injections (7 and 10 months, respectively). At the assessment visit at 3 months after the treatment plan, the first patient had lost 5 lines of BCVA, and CRT on OCT had increased by 214 μm. Fluorescein angiography revealed that the CNV lesion had grown by 1 disc area. In the second patient, BCVA was stable, and CRT only slightly increased by 34 μm, but routine fluorescein angiography revealed a growing lesion. In both instances, we decided to continue with a treatment plan of monthly injections, followed by a progressive interval extension according to the protocol. Within those patients who underwent a 3-month treatment plan after a previous prolonged recurrence interval (ranging from 4 to 16 months; n=27), these two events represented a small proportion (7.4%) as compared with 25 other eyes (92.6%) that showed no severe worsening.
Discussion
In this study, we presented the 2-year outcome of the ‘observe-and-plan’ regimen that consists of an individually tailored treatment plan with ranibizumab for nAMD. The results demonstrated that good functional outcomes may be obtained for up to 2 years with administration of the appropriate number of injections, yet with a dramatically reduced number of ophthalmic examinations, by individual prediction of future treatment needs for up to 6 months. The functional results of this study, particularly the maintenance of the initial visual improvement, may serve as clinical validation of the regimen. The visual results of the ‘observe-and-plan’ regimen are comparable with those from other successful regimens.2, 3, 4, 12, 16, 17, 18 Therefore, we suggest that the individualized prediction of future treatment need, which in this regimen was applied for a maximum of three injections and combined with the dynamic feedback mechanism (assessment visits), provides adequate treatment for nAMD. Interestingly, the functional results of this study were good, although visit delays up to 3 weeks were not excluded from the study. Therefore, the regimen may be well suitable for real-life circumstances.
The ‘observe-and-plan’ regimen allows for elimination of monthly repeated evaluation visits during the treatment plans, thereby reducing the number of ophthalmic assessments to one-third (33%) and one-fourth (24%) during the first and second year, respectively, as compared with the monthly visits required in PRN regimens.11, 12, 16 Thus far, various studies have concluded that strict monthly visits are mandatory in a PRN regimen.17, 19, 20, 21 Correspondingly, it was found that less-than-monthly assessment visits in PRN had a poorer outcome than strict monthly visits.22 However, in a real-life setting, monthly assessments are a logistical problem,23 requiring human resources and time investment from health-care providers as well as patients.13 The treat-and-extend regimen slightly decreased the number of assessment visits (8.4 visits and injections during year 1 (70% of PRN)18) and, because of the ease of planning future injections and visits, it has been widely accepted in clinical routine practice (American Society of Retina Specialists, Preferences and Trends Survey, 2013, available at https://www.asrs.org/content/documents/_2013asrspatsurveyresults.pdf).
The ‘observe-and-plan’ regimen allows for a further reduction in the number of assessment visits to 4.0 and 2.9 in the first and second year, respectively. These examination visits are the time-consuming part of patient care, and their number is determining for the clinical burden. Thus, the ‘observe-and-plan’ regimen improves an institution’s capacity to manage a high number of patients with the given resources, or available resources may be applied elsewhere. ‘Observe-and-plan’ regimen did not aim to further reduce the number of injections, but was designed to anticipate each individual’s need for treatment. The number of injections was very similar between the ‘observe-and-plan’, PRN, and treat-and-extend regimens. Although comparisons between studies are problematic, the number of injections over 2 years was slightly higher in this study (13.6) than in the PRN arms of the CATT and the IVAN trials (12.6 and 13, respectively). This minor difference may be because of the three loading doses and the use of spectral domain OCT with its high sensitivity to detect fluid. Anyhow, several studies have shown that the mean number of injections may not be too much reduced without loss of efficacy.24, 25, 26 The main purpose of this study was to describe a way to apply the individually needed injections with reduced number of visits in order to offer a regimen that would contribute to fight against the real-life danger of undertreatment and visual loss.27
In the absence of monthly evaluation visits, one may be concerned about potential undertreatment in the case of a rapidly changing need for injections. In our 2-year results, we identified one eye with vision loss because of severe exudative recurrence, and another eye with lesion growth. Lesion growth may occur even under monthly fixed regimen.28 However, both events occurred after a 3-month interval treatment plan that had followed a prolonged observation period without treatment. Therefore, we suggest that particular attention should be paid to late recurrences. Delayed exudative recurrence might sometimes—although infrequently (7.4%)—require treatment intervals shorter than 3 months, similar to new CNV development. In an effort to correctly adjust the treatment interval for these rare but aggressive late recurrences, it might be helpful to: (1) consider the severity of the recurrence on OCT, (2) add a monitoring visit at 1.5 months (instead of 2 × 3 months), and (3) encourage the patient to come back early in case of any visual worsening. However, our study showed that only 4% of eyes lost more than three lines of BCVA after 24 months, and this is not above the expected proportion in the reference trials MARINA and ANCHOR (∼10%),2, 4 thereby suggesting good safety of the regimen for visual outcome.
The mean interval increased only slightly from 2.0 months in the first year to 2.2 months in the second year. However, the mean interval values do not take into account the individual variation. For this reason, patients were categorized into short, intermediate, and long intervals in the first and second years (individual average). Most patients remained in their category from year 1 to year 2, and the statistical test showed that overall there was no significant category change. Similarly, the analysis of the first measured interval vs the last applied interval showed that 57% of eyes remained stable over the 2-year course, whereas 31% lengthened their interval by ≥2 steps, and 11% shortened their intervals. The visual results of the study, and particularly the low proportion of patients with visual loss, suggests that the ‘observe-and-plan’ regimen was adequately sensitive to capture this change of retreatment need and to apply sufficient treatment. This is most important for those with need for shorter intervals over time.
Bilateral cases that completed this study were of interest in terms of symmetry between the two eyes. However, only 10 patients (20 eyes) were available for this analysis. Although some showed highly symmetric need for retreatment (4 out of 10), others were quite different. The corresponding statistical test showed a nonsignificant trend towards a difference between the eyes (P=0.063), suggesting that ocular factors may play a more important role than systemic factors. However, no firm conclusion can be drawn with these small numbers.
The evaluation of proportion of eyes with fluid needs to take into account that the ‘observe-and-plan’ strategy would lead to 50% presence of fluid (once in the treatment plan phase), even in case of a perfectly stable injection-recurrence interval. The finding of slightly lower proportions for the last visit before month 12 (46.0%) and month 24 (43.8%) matches well with the slight increase of the mean interval over time, corresponding with a slight decrease of need for retreatment. However, because of the inherent characteristics of the ‘observe-and-plan’ regimen, these numbers cannot be compared with those of a PRN regimen such as the CATT trial, as the visits in this study are planned close to the likely recurrence only and not at a specific time point such as month 12.
The proportion of eyes with fluid present according to the interval groups of short, intermediate, or long intervals showed a statistically equal distribution at the visit before month 12, but a significantly higher proportion with fluid in the short intervals at month 24 (82.1%). This high proportion might be dominated by refractory cases, possibly because of a phenomenon of tachyphylaxia.
We acknowledge that the present study has several weaknesses. It was a single-arm uncontrolled study. The ‘observe-and-plan’ regimen was validated only in that it maintained the VA gain after month 3 through to month 24. The regimen is slightly more complex than the well-known PRN or treat-and-extend regimen. However, in our experience, the involved ophthalmologists were rapidly familiar with the regimen and subjectively did not consider it complex. Both doctor and patient easily understood the regimen through the key concept.
In conclusion, the ‘observe-and-plan’ regimen significantly improved VA and allowed for applying the individually needed number of injections while dramatically reducing the number of assessment visits. Thereby, this regimen alleviates the burden of nAMD management, allowing doctors and institutions to better cope with the chronic care management of nAMD. However, the regimen should ideally be investigated in direct comparison with the gold standard of monthly retreatment to confirm its value.

Change history
12 March 2015
This article has been corrected since advance online publication and a corrigendum is also printed in this issue
References
Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82 (11): 844–851.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1432–1444.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T . Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009; 116 (1): 57–65.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014; 121 (1): 193–201.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012; 119 (12): 2537–2548.
Bloch SB, Larsen M, Munch IC . Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol 2012; 153 (2): 209–213 e202.
Abraham P, Yue H, Wilson L . Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol 2010; 150 (3): 315–324.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008; 145 (2): 239–248.
Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, xer-Siegel R et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011; 118 (5): 831–839.
Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z et al. Twelve-month efficacy and safety of 0.5mg or 2.0mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013; 120 (5): 1046–1056.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364 (20): 1897–1908.
Han DP . Age-related macular degeneration, anti-VEGF therapy, and ophthalmic imaging: is there a best practice? JAMA Ophthalmol 2013; 131 (9): 1124–1126.
Mantel I, Niderprim SA, Gianniou C, Deli A, Ambresin A . Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol 2014; 98 (9): 1192–1196.
Mantel I, Deli A, Iglesias K, Ambresin A . Prospective study evaluating the predictability of need for retreatment with intravitreal ranibizumab for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2013; 251 (3): 697–704.
IVAN Study Investigators Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012; 119 (7): 1399–1411.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD . A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology 2010; 117 (11): 2134–2140.
Haller JA . Current anti-vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology 2013; 120 (5 Suppl): S3–S7.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009; 148 (1): 43–58.
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011; 118 (4): 663–671.
Tschuor P, Pilly B, Venugopal D, Gale RP . Optimising assessment intervals improves visual outcomes in ranibizumab-treated age-related neovascular degeneration: using the stability phase as a benchmark. Graefes Arch Clin Exp Ophthalmol 2013; 251 (10): 2327–2330.
Wolf A, Kampik A . Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol 2014; 252 (4): 647–655.
Dadgostar H, Ventura AA, Chung JY, Sharma S, Kaiser PK . Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology 2009; 116 (9): 1740–1747.
Gerding H . [Treatment of wet AMD with less than 12 injections of Ranibizumab per year]. Klin Monatsbl Augenheilkd 2010; 227 (4): 294–297.
Gerding H . Ranibizumab treatment in age-related macular degeneration: a meta-analysis of one-year results. Klin Monatsbl Augenheilkd 2014; 231 (4): 427–431.
Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014; 121 (5): 1092–1101.
Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011; 118 (3): 523–530.
Acknowledgements
We thank Mrs Mary C Love for her editorial help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Gianniou, C., Dirani, A., Ferrini, W. et al. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration: how to alleviate the clinical burden with maintained functional results. Eye 29, 342–349 (2015). https://doi.org/10.1038/eye.2014.258
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.258
This article is cited by
-
Delaying anti-VEGF therapy during the COVID-19 pandemic: long-term impact on visual outcomes in patients with neovascular age-related macular degeneration
BMC Ophthalmology (2023)
-
A model to quantify the influence of treatment patterns and optimize outcomes in nAMD
Scientific Reports (2022)
-
Modifiable Determinants of Satisfaction with Intravitreal Treatment in Patients with Neovascular Age-Related Macular Degeneration
Drugs & Aging (2022)
-
Erste Erfahrungen mit Brolucizumab bei neovaskulärer altersabhängiger Makuladegeneration und Therapierefraktärität unter der bisherigen Anti-VEGF-Therapie
Der Ophthalmologe (2022)
-
Refractory neovascular age-related macular degeneration: time-dependent changes of central retinal thickness with anti-VEGF treatment
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)