Abstract
Purpose
To evaluate the diagnostic accuracy of spectral-domain optical coherence tomography (SD-OCT) for neovascular age-related macular degeneration (nAMD): a comparison against fundus fluorescein angiography (FFA).
Methods
A retrospective review of SD-OCT, colour fundus photographs (FP), and FFA of 411 consecutive patients referred to a rapid access Macular Clinic over a 4-year period was performed. FFA images were reviewed nonstereoscopically. SD-OCT images were acquired using the Topcon 3D OCT-1000 instrument. All FFA and OCT images were graded by at least two ophthalmologists independently. Side-by-side grading took place with immediate open discussion and adjudication. If there was disagreement between the two grading ophthalmologists or they were not 90% confident of their assigned grade, then adjudication by a third ophthalmologist was performed.
Results
A total of 278 eyes were graded as having choroidal neovascularisation (CNV) with SD-OCT and 231 diagnosed with FFA. The main diagnostic CNV classifications on FFA were: classic no occult in 27 eyes, predominantly classic in 16, minimally classic in 50, occult in 129, and 9 peripapillary membranes. There were a total of 47 false positives with SD-OCT: a rate of 16.9%. The sensitivity and specificity of SD-OCT alone for detecting CNV was 100 and 80.8%, respectively.
Conclusion
Our study confirms SD-OCT in comparison to the reference standard of nonstereoscopic FFA is highly sensitive at detecting newly presenting nAMD in the setting of a specialist AMD clinic where the investigations are interpreted by trained specialists. However, it does not seem accurate enough to replace FFA in the diagnosis on nAMD in current practice.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment in elderly patients across developed countries1 and the third leading cause of global blindness.2 The prevalence of AMD has been reported across a range of populations and measures as high as 25% for the over 75-year age group.3 Stereoscopic fundus fluorescein angiography (FFA) is the current gold standard for the diagnosis of neovascular AMD (nAMD)and its use is recommended by the Royal College of Ophthalmologists in the UK.4 It permits a functional assessment of the retinal and choroidal circulation as well as identification of a breakdown in the blood retinal barrier. The current FFA classification system for nAMD is a historical throwback to the era of laser photocoagulation and photodynamic therapy (PDT) and reflects the need to identify predominantly classic lesions that are eligible for treatment with these now infrequently used therapies.5, 6 Despite the widespread use of FFA, the imaging modality does have some significant drawbacks. It is an invasive procedure, requiring appropriate written consent before it being performed. Serious adverse reactions are extremely rare but are known to occur.7 Yannuzzi et al8 estimated the risk of death following FFA to be 1:222 000. Minor adverse reactions are not uncommon. Patients have to be cannulated, which can be difficult in patients with poor venous access. It is essential that facilities for resuscitation be available. FFA is time-consuming, taking approximately 15–20 min to complete. The quality of the images attained is operator dependent, requiring skill and experience. FFA can be a potential delaying step in the initiation of patients’ treatment, as well as being described at times as ‘inconvenient’, and possibly, unnecessary before commencing therapy with anti-VEGF therapies.
Optical coherence tomography (OCT) was introduced in 1991 and quickly became a widely used imaging technique for a range of ocular diseases that affect the choroid and retina.9 Unlike FFA which is dynamic and detects the presence of leakage, OCT provides 2–3-dimensional structural information on chorioretinal layers and the presence of fluid in the retinal, subretinal, and subpigment epithelial spaces, which are considered a surrogate for leakage. OCT is increasingly used to determine both the presence and activity of choroidal neovascularisation (CNV) and the need for treatment/retreatment in clinical trials and routinely in clinical practice.10, 11 OCT is noninvasive and quick to perform. It has therefore been suggested that OCT imaging may be sufficient in the diagnosis of nAMD, allowing prompt initiation of therapy. A recent systematic analysis has, however, concluded that OCT imaging may not replace but supplement FFA in the diagnosis of nAMD.12 The role of OCT as a diagnostic tool in the setting of a specialist AMD clinic has not been widely investigated. Specifically, there are very few clinical studies to date that have evaluated the accuracy of OCT imaging against the gold standard of stereoscopic FFA for the diagnosis of nAMD.13, 14, 15, 16, 17 Across the studies performed a wide range of different sensitivities and specificities for the detection of CNV have been reported, in part reflecting the mixed use of either the earlier time-domain (TD) or more contemporary spectral domain OCT (SD-OCT) machines and the varied grading methods used. To the best of our knowledge, no publication has assessed the diagnostic accuracy of SD-OCT as compared with nonstereoscopic FFA as the reference standard. We believe that this may reflect a more real-world clinical comparison as many ophthalmologists do not regularly use a stereoscopic viewer while reviewing fluorescein angiograms in their routine clinical practice.
Materials and methods
A retrospective review of all SD-OCT, colour fundus photographs (FP), and FFA of 411 consecutive patients (822 eyes) that were referred to a rapid access Macular Clinic in Kings Mill Hospital, Sutton-in-Ashfield, over a 4-year period (February 2009 to February 2013) was performed. Inclusion criteria were all patients over 50 years of age that were referred for suspected nAMD by optometrists, general practitioners or other ophthalmologists and had symptoms of reduced vision, metamorphopsia, or signs suggestive of nAMD, as determined by the referring clinician. In order to make our results comparable to real-world clinical practice, patients who may have had treatment 6 or more months previously with verteporfin photodynamic therapy or antivascular endothelial growth factor (anti-VEGF) but were thought to have new CNV lesions were included in our study. All individuals were over the age of 50 years. The refractive status of patients was not known. A small number of individuals may have been myopic and these were not excluded from the analysis. Exclusion criteria included all patients that had either no SD-OCT or FP/FFA available for analysis or those patients where one imaging modality was deemed ungradable. In addition, if the SD-OCT or FFA were not performed within 7 days of each other the patient was excluded. Patients with CNV secondary to angioid streaks or evidence of chorioretinitis were excluded. No patients were excluded on the basis of their best corrected visual acuity.
FP was performed using the Topcon TRC-50DX, Type IA retinal camera (Topcon, Tokyo, Japan) combined with an attached Nikon D7000, 16.2 Megapixel camera. The operator was an experienced ophthalmic photographer. Bilateral 35° mydriatic non-stereoscopic photographs of field 2 (centred on the fovea) were reviewed. The standard FFA protocol included 35° images of the transit phase, mid phase, and late phase up to 10 min. Images were all reviewed nonstereoscopically.
SD-OCT images were acquired using the Topcon 3D OCT-1000 instrument (Topcon). The machine was set to perform a 3D scan with a resolution of 512 by 128 and a scan length of 6.0 mm by 6.0 mm. All SD-OCT images were graded by at least two ophthalmologists with appropriate experience in AMD image grading (CW, MP, and AL). SD-OCT images were all reviewed without reference to the FFA or FP. The grader was blind to any clinical patient information such as history, visual acuity, or which eye was the index eye, if not both. Side by side independent grading took place with immediate open discussion and adjudication. If there was disagreement between the two grading ophthalmologists or they were not 90% confident of their assigned grade then adjudication by a third ophthalmologist would take place. After the assignment of the SD-OCT grade, the patient’s mydriatic colour FP was reviewed. Repeat grading of the SD-OCT was performed. It was documented if reference to the FP changed the diagnostic grade assigned.
FFAs were graded by at least two ophthalmologists (CW, AL, and WA) independently but side by side, and blind to both the SD-OCT grade and all clinical information. If disagreement existed or the lesion was questionable then adjudication would take place. There was a temporal delay between the grading of all SD-OCT images and its corresponding FFA of at least 4 weeks.
For FFA, CNV lesions were graded using the Macular Photocoagulation Study grading protocol that was utilised in the treatment of AMD with laser photocoagulation, and photodynamic therapy (TAP) and verteporfin in photodynamic therapy studies.5, 6, 18 Classic CNV was identified as an area of uniform and early (<30 s) hyperfluorescence that showed leakage throughout the mid and late phases. Occult CNV was identified by areas of increasing stippled hyperfluorescence that appeared in the mid and late phases of the FFA with a leak or a late leak of undetermined origin. FFA images were graded as either: classic, predominantly classic, minimally classic, occult, disciform scar, peripapillary CNV, no CNV, or other pathology. CNV was considered present on FFA if classic or occult leakage was detected.
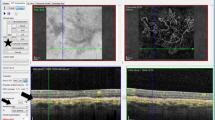
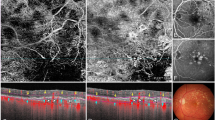
With OCT, CNV was considered present with the grading of changes at the levels of the inner choroid, the RPE or the retina as summarised in Figures 1,2,3,4,5. Other OCT grades were geographic atrophy, drusenoid pigment epithelial detachment (PED), nil CNV, other ocular pathology and disciform scar.
A fusiform or dome shaped area of high reflectivity located within the subretinal space directly adjacent to the presumed retinal pigment epithelium (RPE). This may or may not be associated with sub-retinal fluid (SRF), intra-retinal fluid (IRF), or haemorrhage. Subretinal haemorrhage was ruled out as the cause of this lesion by using point to point correlation between the SD-OCT B scan and the OCT photograph. This utilised PinPoint Registration software.
The presence of a nondrusenoid RPE elevation: a pigment epithelial detachment (PED). If confluent drusen and a PED exist, a drusenoid PED was ruled out by the use of point to point correlation with the B scan and the OCT colour photograph. The PED can range in appearance from a large dome-shaped elevation of RPE with low reflectivity within and a visible Bruch’s membrane, to an irregular and corrugated detachment that may be shallow with moderate reflectivity throughout or on the underside of the RPE. A PED was graded with or without SRF, IRF, or haemorrhage.
Features in keeping with a possible peripapillary CNV or idiopathic polypoidal choroidal vasculopathy were also graded if there were: peripapillary exudate, haemorrhage, PED, retinal thickening, or SRF/IRF that were due to no other identifiable ocular pathology and could be seen to approach the OCT macular grid from the optic disc/nasal direction.
Once all FFA and OCT images had been graded the data was cleaned and any disparities detected were adjudicated and any errors were corrected.
We certify that all applicable institutional and governmental regulations were followed during this research.
Results
A total of 411 patients (822 eyes) were referred to the macular clinic between the dates 02/2009 and 02/2013. All available FFA and OCT images were assessed. Out of these 346 eyes were excluded as they had either no OCT, FFA or had images that were deemed ungradable. Included in this group were 6 eyes that had active CNV identified on both FFA and SD-OCT. However, there were insufficient FFA frames to allow CNV subtype classification. These eyes were excluded from further analysis. A total of 476 eyes had both gradable FFA and SD-OCT. Of these, 198 eyes had no CNV identified with both FFA and OCT; these included 19 eyes classified as having chronic disciform scars, leaving 278 eyes that were graded as having CNV with OCT.
Within this group (of 278 eyes with CNV) the mean age was 80.6 years (SD 4.7), range 51–97. Of these 115 (41.4%) were male, 163 (58.6%) were female. A total of 231 eyes were diagnosed as having CNV with FFA with 32 patients presenting with bilateral CNV as diagnosed with FFA. The main diagnostic CNV classifications on FFA were: classic no occult in 27 eyes, predominantly classic in 16, minimally classic in 50, occult in 129 and 9 peripapillary.
There were a total of 47 false positives with SD-OCT: a rate of 16.9%. Seven of these were diagnosed as disciform scars (ie inactive chronic CNV scars) with FFA. These were graded as either subretinal membranes or PEDs with SD-OCT. The remaining 40 false positives were graded as having features such as a PED, IRF, SRF, or retinal thickening as illustrated in Figures 1,2,3,4,5, but FFA revealed no evidence of active CNV or disciform scar. Reference to the colour fundus photograph did not change any of our SD-OCT grades. There was only one false negative with the use of SD-OCT in the primary grading. This SD-OCT was of a poor quality, and upon primary grading, was believed to have no identifiable features of CNV visible on B scan or OCT photograph. Upon review of the colour FP, peripapillary disc haemorrhages were noted and FFA revealed an occult lesion. However, during adjudication the SD-OCT was reviewed and nasal retinal thickening was in fact present but missed as part of a grading error. This haemorrhage was not visible on the OCT photograph. The sensitivity and specificity of SD-OCT alone for detecting CNV was 100% and 80.8%, respectively. Amongst the eyes graded as not having any evidence of CNV on OCT and FFA, there were 77 eyes that were found to have other ocular pathology identified as the primary diagnosis.
Discussion
SD-OCT is increasingly used in clinical practice for the follow-up of patients undergoing treatment with anti-VEGF in nAMD and other retinal vascular disease. It provides a time efficient, noninvasive imaging tool that allows high-resolution, pseudohistological cross-sectional images of the retina, RPE, and choroid. It has been suggested that OCT may similarly be used as the primary diagnostic tool in the management of nAMD therefore reducing the requirement of FFA. The present study, however, agrees with the conclusions of the recent systematic review, that SD-OCT cannot yet replace FFA as the gold standard in the diagnosis of nAMD.12
Our study confirms that SD-OCT in comparison to the reference standard of non-stereoscopic FFA is highly sensitive (100%) at detecting newly presenting nAMD in the setting of a specialist AMD clinic where the investigations are interpreted by trained specialists. However, it does not seem accurate enough yet to replace or equate FFA in the diagnosis on nAMD. The specificity of SD-OCT in our study is comparable to those previously reported elsewhere,13, 16 despite the higher sensitivity than that obtained in some previous reports.13, 15, 16 There are several explanations for this including the use of different grading criteria, differing patient populations and, of course, diagnostic imaging technologies utilised. Sandhu et al13reported the sensitivity with TD-OCT alone to be 96.4%. The use of TD-OCT has been reported by others to fail frequently in the detection of abnormalities that are associated with fluorescein leakage from CNV on FFA19 and a few studies have demonstrated SD-OCT to be more sensitive at detecting abnormalities associated with nAMD than TD-OCT.20, 21, 22 This will, in part, explain the improved sensitivity with our results. Another recent publication investigating new patients referred for suspected wet AMD also reported a sensitivity of 100%.14The lower sensitivity reported by others such as Khurana et al22 may reflect their study population. In that particular example 80% of assessed eyes had previously been treated with anti-VEGF. Other differences in reported sensitivities may reflect the variations in grading the OCT images and the inconsistency of definitions used for diagnosis. An example is the AMD Doc Study15 that reported a sensitivity of 69% in the detection of conversion to nAMD in high risk eyes. The criteria used for the detection of CNV with SD-OCT primarily involved assessing retinal changes that occurred secondary to a breakdown in the outer blood–retinal barrier such as the presence of subretinal fluid, a 10% increase in retinal thickening or intraretinal cystic abnormalities.15 Other studies like ours and that by Sandhu et al13 have graded OCT images for the morphological features of the fibrovascular complex19, 23 in addition to their exudative consequences of retinal thickening and oedema as opposed to just the latter. In 5% of eyes in our study no SRF or IRF was present on SD-OCT, yet FFA demonstrated an occult lesion. In all of these cases SD-OCT demonstrated a PED. This highlights the importance of grading PEDs in the absence of IRF/SRF to avoid unnecessary false negatives. However, it is accepted that the mere presence of PEDs does not imply an active CNV. During the primary grading in the present study there was only one false negative with the use of SD-OCT, which on adjudication turned out to be a grading error as explained earlier. This highlights another possible limitation of SD-OCT for the imaging of nAMD. Given the small field (6 mm × 6 mm) used for routine scans of the macular area, eccentric pathology, and peripapillary lesions could be missed that would otherwise be detected with standard protocols within the larger 35° field of FFA. In the future as larger SD-OCT fields become more routinely used this problem may be overcome. The Topcon 3D OCT 1000 (Topcon) used in this study can image an area of 8.2 mm which would have included both the disc and the macular area and may have prevented this grading error. However, the imaging protocol did not include the disc and nasal peripapillary zones.
The high false-positive rate in the present study represents the difficulty in correctly identifying areas of hyper-reflectivity on SD-OCT that represent active CNV, and distinguishing them from those that represent inactive gliosis, particularly in the setting of chronic lesions. SD-OCT, not unexpectedly, seems to allow easy identification of structural changes that indicate there has been a previous or currently active CNV. However, it is unable to determine whether the fluid detected is from an active CNV at diagnosis. Furthermore, other causes of intraretinal cysts or fluid do occur and may confound diagnosis of CNV based purely on the presence of such spaces. An alternative possible explanation for some of these false positive cases, however, is that obscuring lesions such as a staining scar could have covered and masked any late leak deep to the scar on FFA. The cause of the discrepancies where SD-OCT demonstrates a PED without disruption of the RPE, or the presence of SRF/IRF but FFA demonstrates no CNV is not known at the present time, and definitely warrants further investigation. It has previously been demonstrated that second eye conversion to nAMD is unpredictable in only a small number of patients (12%), but that in the majority of cases, changes such as IRF, or SRF were preceded by the development of RPE elevation.24 In other cases, the association of subretinal space, presumed as subretinal fluid, with PED may represent nothing more than potential space and contain no leaking fluid at all. The presence of intraretinal cysts may represent intraretinal changes secondary to chronic disease processes other than neovascular AMD such as retinal dystrophy or other degeneration.
Alternatively, although unlikely, the findings may reflect SD-SOCT as being a more sensitive imaging modality than the presumed gold standard and these changes may in some eyes represent the very early structural changes that occur prior to a detectable leak with FFA.24 In addition, the FFAs were limited to 10 min as in standard practice so that there is a chance that late leakage of undetermined origin is missed. It is important to note that SD-OCT images as well as the FFA were interpreted by persons with expertise in the technologies. It would be expected that sensitivity would be lower and false-positive rates higher amongst those with less expertise.
In conclusion, the current study confirms that SD-OCT has a high sensitivity in detecting nAMD. However, specificity and false-positive rates are currently unacceptable when compared with FFA. As such SD-OCT cannot replace FFA in the diagnosis of nAMD in current clinical practice.

References
Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122 (4): 477–485.
Pascolini D, Mariotti SP . Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96 (5): 614–618.
Klein R, Peto T, Bird A, Vannewkirk MR . The epidemiology of age-related macular degeneration. Am J Ophthalmol 2004; 137 (3): 486–495.
Chakravarthy U Age-Related Macular Degeneration:Guidelines for Management: Royal College of Ophthalmologists; 2013 [cited November 2013]. Available at http://rcophth.ac.uk/page.asp?section=451.
Barbazetto I, Burdan A, Bressler NM, Bressler SB, Haynes L, Kapetanios AD et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment—TAP and VIP report No. 2. Arch Ophthalmol 2003; 121 (9): 1253–1268.
MPSG. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Macular Photocoagulation Study Group. Arch Ophthalmol 1991; 109 (9): 1242–1257.
Kwan AS, Barry C, McAllister IL, Constable I . Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience. Clin Exp Ophthalmol 2006; 34 (1): 33–38.
Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W et al. Fluorescein angiography complication survey. Ophthalmology 1986; 93 (5): 611–617.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W et al. Optical coherence tomography. Science 1991; 254 (5035): 1178–1181.
Kaiser PK, Blodi BA, Shapiro H, Acharya NR, Group MS. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007; 114 (10): 1868–1875.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Castillo MM, Mowatt G, Lois N, Elders A, Fraser C, Amoaku W et al. Optical coherence tomography for the diagnosis of neovascular age-related macular degeneration: a systematic review. Eye 2014; 28 (12): 1399–1406.
Sandhu SS, Talks SJ . Correlation of optical coherence tomography, with or without additional colour fundus photography, with stereo fundus fluorescein angiography in diagnosing choroidal neovascular membranes. Br J Ophthalmol 2005; 89 (8): 967–970.
Talks J, Koshy Z, Chatzinikolas K . Use of optical coherence tomography, fluorescein angiography and indocyanine green angiography in a screening clinic for wet age-related macular degeneration. Br J Ophthalmol 2007; 91 (5): 600–601.
Do DV, Gower EW, Cassard SD, Boyer D, Bressler NM, Bressler SB et al. Detection of new-onset choroidal neovascularization using optical coherence tomography: the AMD DOC Study. Ophthalmology 2012; 119 (4): 771–778.
Mokwa NF, Ristau T, Keane PA, Kirchhof B, Sadda SR, Liakopoulos S . Grading of age-related macular degeneration: comparison between color fundus photography, fluorescein angiography, and spectral domain optical coherence tomography. J Ophthalmol 2013; 2013: 385915.
Padnick-Silver L, Weinberg AB, Lafranco FP, Macsai MS . Pilot study for the detection of early exudative age-related macular degeneration with optical coherence tomography. Retina 2012; 32 (6): 1045–1056.
Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993; 111 (9): 1200–1209.
Eter N, Spaide RF . Comparison of fluorescein angiography and optical coherence tomography for patients with choroidal neovascularization after photodynamic therapy. Retina 2005; 25 (6): 691–696.
Sayanagi K, Sharma S, Yamamoto T, Kaiser PK . Comparison of spectral-domain versus time-domain optical coherence tomography in management of age-related macular degeneration with ranibizumab. Ophthalmology 2009; 116 (5): 947–955.
Keane PA, Bhatti RA, Brubaker JW, Liakopoulos S, Sadda SR, Walsh AC . Comparison of clinically relevant findings from high-speed fourier-domain and conventional time-domain optical coherence tomography. Am J Ophthalmol 2009; 148 (2): 242–8 e1.
Khurana RN, Dupas B, Bressler NM . Agreement of time-domain and spectral-domain optical coherence tomography with fluorescein leakage from choroidal neovascularization. Ophthalmology 2010; 117 (7): 1376–1380.
Sulzbacher F, Kiss C, Munk M, Deak G, Sacu S, Schmidt-Erfurth U . Diagnostic evaluation of type 2 (classic) choroidal neovascularization: optical coherence tomography, indocyanine green angiography, and fluorescein angiography. Am J Ophthalmol 2011; 152 (5): 799–806 e1.
Amissah-Arthur KN, Panneerselvam S, Narendran N, Yang YC . Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye 2012; 26 (3): 394–399.
Acknowledgements
This work was part funded by a research grant from the Macular Society UK. The views expressed are those of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wilde, C., Patel, M., Lakshmanan, A. et al. The diagnostic accuracy of spectral-domain optical coherence tomography for neovascular age-related macular degeneration: a comparison with fundus fluorescein angiography. Eye 29, 602–610 (2015). https://doi.org/10.1038/eye.2015.44
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.44
This article is cited by
-
Macular neovascularization lesion type and vision outcomes in neovascular age-related macular degeneration: post hoc analysis of HARBOR
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Optical coherence tomography angiography for detection of macular neovascularization associated with atrophy in age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Prevalence of peripapillary choroidal neovascular membranes (PPCNV) in an elderly UK population—the Bridlington eye assessment project (BEAP): a cross-sectional study (2002–2006)
Eye (2019)
-
The possibility of the combination of OCT and fundus images for improving the diagnostic accuracy of deep learning for age-related macular degeneration: a preliminary experiment
Medical & Biological Engineering & Computing (2019)
-
Sensitivity and specificity of optical coherence tomography angiography (OCT-A) for detection of choroidal neovascularization in real-life practice and varying retinal expertise level
International Ophthalmology (2018)