Abstract
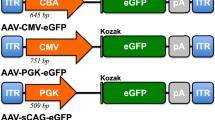
Specific cone-directed therapy is of high priority in the treatment of human hereditary retinal diseases. However, not much information exists about the specific targeting of photoreceptor subclasses. Three versions of the human red cone opsin promoter (PR0.5, 3LCR-PR0.5 and PR2.1), and the human blue cone opsin promoter HB569, were evaluated for their specificity and robustness in targeting green fluorescent protein (GFP) gene expression to subclasses of cones in the canine retina when used in recombinant adeno-associated viral vectors of serotype 5. The vectors were administered by subretinal injection. The promoter PR2.1 led to most effective and specific expression of GFP in the long- and medium-wavelength-absorbing cones (L/M cones) of normal and diseased retinas. The PR0.5 promoter was not effective. Adding three copies of the 35-bp LCR in front of PR0.5 lead to weak GFP expression in L/M cones. The HB569 promoter was not specific, and GFP was expressed in a few L/M cones, some rods and the retinal pigment epithelium. These results suggest that L/M cones, the predominant class of cone photoreceptors in the retinas of dogs and most mammalian species can be successfully targeted using the human red cone opsin promoter.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kohl S, Marx T, Giddings I, Jägle H, Jacobson SG, Apfelstedt-Sylla E et al. Total colourblindness is caused by mutations in the gene encoding the α-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet 1998; 19: 257–259.
Kohl S, Baumann B, Rosenberg T, Kellner U, Lorenz B, Vadalà M et al. Mutations in the cone photoreceptor G-protein α -subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet 2002; 71: 422–425.
Sundin OH, Yang JM, Li Y, Zhu D, Hurd JN, Mitchell TN et al. Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet 2000; 25: 289–293.
Sidjanin DJ, Lowe JK, McElwee JL, Milne BS, Phippen TM, Sargan DR et al. Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet 2002; 11: 1823–1833.
Demirci FY, Gupta N, Radak AL, Rigatti BW, Mah TS, Milam AH et al. Histopathologic study of X-linked cone-rod dystrophy (CORDX1) caused by a mutation in the RPGR exon ORF15. Am J Ophthalmol 2005; 139: 386–388.
Beltran WA, Hammond P, Acland GM, Aguirre GD . A frameshift mutation in RPGR exon ORF15 causes photoreceptor degeneration and inner retina remodeling in a model of X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci 2006; 47: 1669–1681.
Ebenezer ND, Michaelides M, Jenkins SA, Audo I, Webster AR, Cheetham ME et al. Identification of novel RPGR ORF15 mutations in X-linked progressive cone-rod dystrophy (XLCORD) families. Invest Ophthalmol Vis Sci 2005; 46: 1891–1898.
Johnson PT, Lewis GP, Talaga KC, Brown MN, Kappel PJ, Fisher SK et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci 2003; 44: 4481–4488.
Léveillard T, Mohand-Saïd S, Lorentz O, Hicks D, Fintz AC, Clérin E et al. Identification and characterization of rod-derived cone viability factor. Nat Genet 2004; 36: 755–759.
Curcio CA, Sloan KR, Kalina RE, Henrickson AE . Human photoreceptor topography. J Comp Neurol 1990; 292: 497–523.
Jacobs GH . The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc 1993; 68: 413–471.
Jacobs GH, Deegan JF, Crognale MA, Fenwick JA . Photopigments of dogs and foxes and their implications for canid vision. Vis Neurosci 1993; 10: 173–180.
Neitz J, Geist T, Jacobs GH . Color vision in the dog. Vis Neurosci 1989; 3: 119–125.
Aguirre GD, Acland GM . Models, mutants and man: searching for unique phenotypes and genes in the dog model of inherited retinal degeneration. In: Ostrander EA, Giger U, Lindblad-Toh K (eds). The Dog and Its Genome. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, 2006, pp 291–325.
Aguirre GD, Rubin LF . Pathology of hemeralopia in the Alaskan malamute dog. Invest Ophthalmol 1974; 13: 231–235.
Aguirre GD, Rubin LF . The electroretinogram in dogs with inherited cone degeneration. Invest Ophthalmol 1975; 14: 840–847.
Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jägle H et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet 2005; 13: 302–308.
Zhang Q, Acland GM, Wu WX, Johnson JL, Pearce-Kelling S, Tulloch B et al. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet 2002; 11: 993–1003.
Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron 1992; 9: 429–440.
Shaaban SA, Deeb SS . Functional analysis of the promoters of the human red and green visual pigment genes. Invest Ophthalmol Vis Sci 1998; 39: 885–896.
Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med 2007; 13: 685–687.
Li Q, Timmers AM, Guy J, Pang J, Hauswirth WW . Cone-specific expression using a human red opsin promoter in recombinant AAV. Vision Res 2008; 48: 332–338.
Mancuso K, Hendrickson AE, Connor TB, Mauck MC, Kinsella JJ, Hauswirth WW et al. Recombinant adeno-associated virus targets passenger gene expression to cones in primate retina. J Opt Soc Am A Opt Image Sci Vis 2007; 24: 1411–1416.
Glushakova LG, Timmers AM, Pang J, Teusner JT, Hauswirth WW . Human blue-opsin promoter preferentially targets reporter gene expression to rat s-cone photoreceptors. Invest Ophthalmol Vis Sci 2006; 47: 3505–3513.
Gropp KE, Szél A, Huang JC, Acland GM, Farber DB, Aguirre GD . Selective absence of cone outer segment beta 3-transducin immunoreactivity in hereditary cone degeneration (cd). Exp Eye Res 1996; 63: 285–296.
Aguirre G, Farber D, Lolley R, Fletcher RT, Chader GJ . Rod-cone dysplasia in Irish Setters: a defect in cyclic GMP metabolism in visual cells. Science 1978; 201: 1133–1134.
Ray K, Baldwin VJ, Acland GM, Blanton SH, Aguirre GD . Cosegregation of codon 807 mutation of the rod cGMP phosphodiesterase β gene and rcd1. Invest Ophthalmol Vis Sci 1994; 35: 4291–4299.
Komáromy AM, Varner SE, de Juan E, Acland GM, Aguirre GD . Application of a new subretinal injection device in the dog. Cell Transplant 2006; 15: 511–519.
Berns KI, Linden RM . The cryptic life style of adeno-associated virus. Bioessays 1995; 17: 237–245.
Dinculescu A, Glushakova L, Min SH, Hauswirth WW . Adeno-associated virus-vectored gene therapy for retinal disease. Hum Gene Ther 2005; 16: 649–663.
Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 2001; 10: 3075–3081.
Auricchio A . Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vision Res 2003; 43: 913–918.
Yang GS, Schmidt M, Yan Z, Lindbloom JD, Harding TC, Donahue BA et al. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J Virol 2002; 76: 7651–7660.
Lotery AJ, Yang GS, Mullins RF, Russell SR, Schmidt M, Stone EM et al. Adeno-associated virus type 5: transduction efficiency and cell-type specificity in the primate retina. Hum Gene Ther 2003; 14: 1663–1671.
Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol 1991; 312: 610–624.
Zolotukhin S . Production of recombinant adeno-associated virus vectors. Hum Gene Ther 2005; 16: 551–557.
Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 2002; 28: 158–167.
Ray K, Tejero MD, Baldwin VJ, Aguirre GD . An improved diagnostic test for rod cone dysplasia 1 (rcd1) using allele-specific polymerase chain reaction. Curr Eye Res 1996; 15: 583–587.
Acknowledgements
This study was supported by NIH Grants EY06855, EY07132, EY11123, EY13132, K12-EY15398, P30-EY01583, NS36302, Foundation Fighting Blindness, Macular Vision Research Foundation and The ONCE International Price for R&D in Biomedicine and New Technologies for the Blind. We thank Jeremy Nathans (Johns Hopkins University—Howard Hughes Medical Institute) for providing the pR2.1-LacZ plasmid; W Clay Smith (University of Florida) for the GFP antibody; Tom Doyle and Min Ding (University of Florida) for assistance in vector production; T Michael Redmond (National Eye Institute, Bethesda, MD, USA) for the RPE65 antibody; Barbara Zangerl (University of Pennsylvania) for the promoter alignments; Amanda Nickle, Gerri Antonini, Alice Eidsen, Tracy Greiner and the staff of the Retinal Disease Studies Facility at the University of Pennsylvania for their technical support; and Mary Leonard for the figures. WWH and the University of Florida have a financial interest in the use of rAAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. All remaining authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komáromy, A., Alexander, J., Cooper, A. et al. Targeting gene expression to cones with human cone opsin promoters in recombinant AAV. Gene Ther 15, 1049–1055 (2008). https://doi.org/10.1038/gt.2008.32
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.32
Keywords
This article is cited by
-
Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye
Pharmaceutical Research (2019)
-
Gentherapie zur Behandlung von Netzhauterkrankungen
Medizinische Genetik (2017)
-
Transient Photoreceptor Deconstruction by CNTF Enhances rAAV-Mediated Cone Functional Rescue in Late Stage CNGB3-Achromatopsia
Molecular Therapy (2013)