Abstract
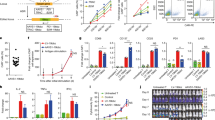
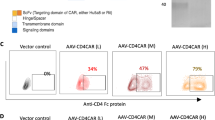
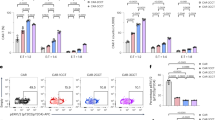
Cancer therapy with T cells expressing chimeric antigen receptors (CARs) has produced remarkable clinical responses in recent trials, but also severe side effects. Whereas most protocols use permanently reprogrammed T cells, we have developed a platform for transient CAR expression by mRNA electroporation. This approach may be useful for safe clinical testing of novel receptors, or when a temporary treatment period is desirable. Herein, we investigated therapy with transiently redirected T cells in vitro and in a xenograft mouse model. We constructed a series of CD19-specific CARs with different spacers and co-stimulatory domains (CD28, OX40 or CD28-OX40). The CAR constructs all conferred T cells with potent CD19-specific activity in vitro. Unexpectedly, the constructs incorporating a commonly used IgG1-CH2CH3 spacer showed lack of anti-leukemia activity in vivo and induced severe, partly CD19-independent toxicity. By contrast, identical CAR constructs without the CH2-domain eradicated leukemia in vivo, without notable toxicity. Follow-up studies demonstrated that the CH2CH3-spacer bound soluble mouse Fcγ-receptor I and mediated off-target T-cell activation towards murine macrophages. Our findings highlight the importance of non-signalling CAR elements and of in vivo studies. Finally, the results show that transiently redirected T cells control leukemia in mice and support the rationale for developing an mRNA-CAR platform.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kochenderfer JN, Rosenberg SA . Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10: 267–276.
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3: 95ra73.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra138.
Jensen MC, Riddell SR . Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev 2014; 257: 127–144.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–2720.
Pule M, Finney H, Lawson A . Artificial T-cell receptors. Cytotherapy 2003; 5: 211–226.
Chmielewski M, Hombach AA, Abken H . CD28 cosignalling does not affect the activation threshold in a chimeric antigen receptor-redirected T-cell attack. Gene Ther 2011; 18: 62–72.
Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK . Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 2006; 20: 1819–1828.
Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK . A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 2005; 12: 933–941.
Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M . Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 2002; 20: 70–75.
Hinrichs CS, Restifo NP . Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol 2013; 31: 999–1008.
Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011; 19: 620–626.
Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013; 21: 904–912.
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013; 36: 133–151.
Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122: 863–871.
Stauss HJ, Morris EC . Immunotherapy with gene-modified T cells: limiting side effects provides new challenges. Gene Ther 2013; 20: 1029–1032.
Westwood JA, Murray WK, Trivett M, Shin A, Neeson P, MacGregor DP et al. Absence of retroviral vector-mediated transformation of gene-modified T cells after long-term engraftment in mice. Gene Ther 2008; 15: 1056–1066.
Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M et al. Resistance of mature T cells to oncogene transformation. Blood 2008; 112: 2278–2286.
Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012; 4: 132ra153.
Almasbak H, Rian E, Hoel HJ, Pule M, Walchli S, Kvalheim G et al. Transiently redirected T cells for adoptive transfer. Cytotherapy 2011; 13: 629–640.
Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther 2011; 22: 1575–1586.
Birkholz K, Hombach A, Krug C, Reuter S, Kershaw M, Kampgen E et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther 2009; 16: 596–604.
Rabinovich PM, Komarovskaya ME, Wrzesinski SH, Alderman JL, Budak-Alpdogan T, Karpikov A et al. Chimeric receptor mRNA transfection as a tool to generate antineoplastic lymphocytes. Hum Gene Ther 2009; 20: 51–61.
Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther 2006; 13: 151–159.
Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y . Regimen-Specific Effects of RNA-Modified Chimeric Antigen Receptor T Cells in Mice with Advanced Leukemia. Hum Gene Ther 2013; 24: 717–727.
Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G et al. Mesothelin-specific Chimeric Antigen Receptor mRNA-Engineered T cells Induce Anti-Tumor Activity in Solid Malignancies. Cancer Immunol Res 2014; 2: 112–120.
Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118: 4817–4828.
Brentjens RJ, Curran KJ . Novel cellular therapies for leukemia: CAR-modified T cells targeted to the CD19 antigen. Hematology Am Soc Hematol Educ Program 2012; 2012: 143–151.
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011; 121: 1822–1826.
Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010; 16: 1245–1256.
Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116: 4099–4102.
Cooper LJ, Jena B, Bollard C.M . Good T cells for bad B cells. Blood 2012; 119: 2700–2702.
Hombach A, Abken H . Costimulation tunes tumor-specific activation of redirected T cells in adoptive immunotherapy. Cancer Immunol Immunother 2007; 56: 731–737.
Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 2013; 19: 3153–3164.
Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol 2008; 180: 4901–4909.
Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 2005; 28: 203–211.
Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE . Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther 2010; 10: 77–90.
Hogarth PM, Pietersz GA . Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Dis 2012; 11: 311–331.
Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK et al. Crosstalk between human IgG isotypes and murine effector cells. J Immunol 2012; 189: 3430–3438.
Hombach A, Hombach AA, Abken H . Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc 'spacer' domain in the extracellular moiety of chimeric antigen receptors avoids 'off-target' activation and unintended initiation of an innate immune response. Gene Ther 2010; 17: 1206–1213.
Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C et al. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res e-pub ahead of print 11 September 2014.
Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012; 119: 3940–3950.
Barrett DM, Seif AE, Carpenito C, Strong EP, June CH, Grupp S.A et al. Bioluminescent tracking of human and mouse acute lymphoblastic leukemia reveals potent immunogenicity of luciferase in some preclinical models of leukemia. 52nd ASH Annual Meeting 4–7 December 2010; Orlando, FL, USA, 2010.
Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 2012; 120: 5185–5187.
Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 2013; 123: 594–599.
Chmielewski M, Kopecky C, Hombach AA, Abken H . IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011; 71: 5697–5706.
Kuhn AN, Diken M, Kreiter S, Selmi A, Kowalska J, Jemielity J et al. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther 2010; 17: 961–971.
Heemskerk B, Jorritsma A, Gomez-Eerland R, Toebes M, Haanen JB, Schumacher TN . Microbead-assisted retroviral transduction for clinical application. Hum Gene Ther 2010; 21: 1335–1342.
Zheng Z, Chinnasamy N, Morgan RA . Protein L: a novel reagent for the detection of chimeric antigen receptor (CAR) expression by flow cytometry. J Transl Med 2012; 10: 29.
Berntzen G, Lunde E, Flobakk M, Andersen JT, Lauvrak V, Sandlie I . Prolonged and increased expression of soluble Fc receptors, IgG and a TCR-Ig fusion protein by transiently transfected adherent 293E cells. J Immunol Methods 2005; 298: 93–104.
Loew R, Heinz N, Hampf M, Bujard H, Gossen M . Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol 2010; 10: 81.
Acknowledgements
This work was supported by The Norwegian Cancer Society, Radiumhospitalets legater, The Norwegian Health Region South East and the Research Council of Norway. We would like to thank Professor I Sandlie (University of Oslo and OUH) for valuable advice and for providing soluble FcγRs. We also thank Dr AA Tveita (OUH) for providing mouse macrophages and Ms. HJ Hoel, Ms. A Faane and Dr N Westerdaal (OUH) for laboratory assistance. We further thank Dr R Löw (EUFETS AG) for providing the reporter vector and LifeTechnologies for supplying M-450 Epoxy and CD3CD28 Dynabeads. Finally, we would like to thank Dr A Kuhn (BioNTech AG) for valuable advice and Dr C Rössig (University Children's Hospital Münster) and Dr M Pule (University College London) for providing CAR plasmids.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Almåsbak, H., Walseng, E., Kristian, A. et al. Inclusion of an IgG1-Fc spacer abrogates efficacy of CD19 CAR T cells in a xenograft mouse model. Gene Ther 22, 391–403 (2015). https://doi.org/10.1038/gt.2015.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.4
This article is cited by
-
Structural engineering of chimeric antigen receptors targeting HLA-restricted neoantigens
Nature Communications (2021)
-
Evolution of chimeric antigen receptor (CAR) T cell therapy: current status and future perspectives
Archives of Pharmacal Research (2019)
-
Preclinical assessment of transiently TCR redirected T cells for solid tumour immunotherapy
Cancer Immunology, Immunotherapy (2019)
-
Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR
Nature Medicine (2019)
-
Turning the tables on cytomegalovirus: targeting viral Fc receptors by CARs containing mutated CH2–CH3 IgG spacer domains
Journal of Translational Medicine (2018)