Abstract
Background/Objectives:
Adipose tissue (AT) autophagy gene expression is elevated in human obesity, correlating with increased metabolic risk, but mechanistic links between the two remain unclear. Thus, the objective of this study was to assess whether elevated autophagy may cause AT endocrine dysfunction, emphasizing the putative role of adiponectin in fat–liver endocrine communication.
Subjects/Methods:
We utilized a large (N=186) human AT biobank to assess clinical associations between human visceral AT autophagy genes, adiponectin and leptin, by multivariate models. A broader view of adipocytokines association with elevated autophagy was assessed using adipocytokine array. Finally, to establish causality, ex vivo studies utilizing a murine AT–hepatocyte cell line co-culture system was used.
Results:
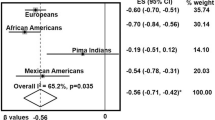
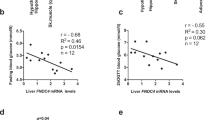
Circulating high-molecular-weight adiponectin and leptin levels were associated with human omental-AT expression of ATG5 mRNA, associations that remained significant (β=−0.197, P=0.011; β=0.267, P<0.001, respectively) in a multivariate model adjusted for age, sex, body mass index and interleukin-6 (IL-6). A similar association was observed with omental-AT LC3A mRNA levels. Bafilomycin-A1 (Baf A) pretreatment of AT explants from high-fat-fed (HFF) mice had no effect on the secretion of some AT-derived endocrine factors, but partially or fully reversed obesity-related changes in secretion of a subset of adipocytokines by >30%, including the obesity-associated upregulation of IL-6, vascular endothelial growth factor, tumor necrosis factor alpha (TNFα) and certain insulin-like growth factor-binding proteins, and the HFF-induced downregulated secretion of IL-10 and adiponectin. Similarly, decreased adiponectin and increased leptin secretion from cultured adipocytes stimulated with TNFα+IL-1β was partially reversed by small interfering RNA-mediated knockdown of ATG7. AT explants from HFF mice co-cultured with Hepa1c hepatoma cells impaired insulin-induced Akt and GSK3 phosphorylation. This effect was significantly reversed by pretreating explants with Baf A, but not if adiponectin was immunodepleted from the conditioned media.
Conclusions:
Reduced secretion of adiponectin may link obesity-associated elevated AT autophagy/lysosomal activity with adipose endocrine dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stienstra R, Haim Y, Riahi Y, Netea M, Rudich A, Leibowitz G . Autophagy in adipose tissue and the beta cell: implications for obesity and diabetes. Diabetologia 2014; 57: 1505–1516.
Maixner N, Kovsan J, Harman-Boehm I, Bluher M, Bashan N, Rudich A . Autophagy in adipose tissue. Obes Facts 2012; 5: 710–721.
Yang L, Li P, Fu S, Calay ES, Hotamisligil GS . Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 2010; 11: 467–478.
Kosacka J, Kern M, Klöting N, Paeschke S, Rudich A, Haim Y et al. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol Cell Endocrinol 2015; 409: 21–32.
Kovsan J, Bluher M, Tarnovscki T, Kloting N, Kirshtein B, Madar L et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab 2011; 96: E268–E277.
Ost A, Svensson K, Ruishalme I, Brannmark C, Franck N, Krook H et al. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med 2010; 16: 235–246.
Nunez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B et al. Defective regulation of adipose tissue autophagy in obesity. Int J Obes 2013; 37: 1473–1480.
Jansen HJ, van Essen P, Koenen T, Joosten LA, Netea MG, Tack CJ et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology 2012; 153: 5866–5874.
Yoshizaki T, Kusunoki C, Kondo M, Yasuda M, Kume S, Morino K et al. Autophagy regulates inflammation in adipocytes. Biochem Biophys Res Commun 2012; 417: 352–357.
Soussi H, Reggio S, Alili R, Prado C, Mutel S, Pini M et al. DAPK2 downregulation associates with attenuated adipocyte autophagic clearance in human obesity. Diabetes 2015; 64: 3452–3463.
Goldman S, Zhang Y, Jin S . Autophagy and adipogenesis: implications in obesity and type II diabetes. Autophagy 2010; 6: 179–181.
Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 2009; 119: 3329–3339.
Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S . Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA 2009; 106: 19860–19865.
Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW Jr . Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab 2013; 18: 816–830.
Kovsan J, Osnis A, Maissel A, Mazor L, Tarnovscki T, Hollander L et al. Depot-specific adipocyte cell lines reveal differential drug-induced responses of white adipocytes–relevance for partial lipodystrophy. Am J Physiol Endocrinol Metab 2009; 296: E315–E322.
Haim Y, Bluher M, Slutsky N, Goldstein N, Kloting N, Harman-Boehm I et al. Elevated adipose tissue autophagy gene expression in humans: a potential non-cell-cycle-dependent function of E2F1. Autophagy 2015; 11: 2074–2088.
Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E et al. Interleukin-1beta regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PloS ONE 2013; 8: e53626.
Nov O, Kohl A, Lewis EC, Bashan N, Dvir I, Ben-Shlomo S et al. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology 2010; 151: 4247–4256.
Bashan N, Dorfman K, Tarnovscki T, Harman-Boehm I, Liberty IF, Bluher M et al. Mitogen-activated protein kinases, inhibitory-kappaB kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology 2007; 148: 2955–2962.
Bluher M, Bashan N, Shai I, Harman-Boehm I, Tarnovscki T, Avinaoch E et al. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body Insulin sensitivity. J Clin Endocrinol Metab 2009; 94: 2507–2515.
Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 2007; 92: 2240–2247.
Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y et al. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab 2013; 98: 1173–1181.
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8: 445–544.
Choi AM, Ryter SW, Levine B . Autophagy in human health and disease. N Engl J Med 2013; 368: 651–662.
Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PloS ONE 2008; 3: e3316.
Zhou L, Liu F . Autophagy: roles in obesity-induced ER stress and adiponectin downregulation in adipocytes. Autophagy 2010; 6: 1196–1197.
Acknowledgements
This study was supported in part by grants from Deutsche Forschungsgemeinschaft (DFG): SFB 1052/1: ‘Obesity mechanisms’ (projects B1 and B2 to AR and MB), and by the Israel Science Foundation (ISF 928/14, to AR). AR is Chair of the Fraida Foundation in Diabetes Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Slutsky, N., Vatarescu, M., Haim, Y. et al. Decreased adiponectin links elevated adipose tissue autophagy with adipocyte endocrine dysfunction in obesity. Int J Obes 40, 912–920 (2016). https://doi.org/10.1038/ijo.2016.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.5
This article is cited by
-
Understanding the molecular mechanisms and role of autophagy in obesity
Molecular Biology Reports (2021)
-
A single bout of resistance exercise improves postprandial lipid metabolism in overweight/obese men with prediabetes
Diabetologia (2020)
-
Up-regulated autophagy: as a protective factor in adipose tissue of WOKW rats with metabolic syndrome
Diabetology & Metabolic Syndrome (2018)
-
Targeting autophagy in obesity: from pathophysiology to management
Nature Reviews Endocrinology (2018)
-
Contributions of the Bone Microenvironment to Monoclonal Gammopathy of Undetermined Significance Pathogenesis
Current Osteoporosis Reports (2018)