Abstract
Desbuquois dysplasia (DBQD) is an autosomal recessive skeletal disorder characterized by growth retardation, joint laxity, short extremities, and progressive scoliosis. DBQD is classified into two types based on the presence (DBQD1) or absence (DBQD2) of characteristic hand abnormalities. CANT1 mutations have been reported in both DBQD1 and DBQD2. Recently, mutations in the gene encoding xylosyltransferase 1 (XYLT1) were identified in several families with DBQD2. In this study, we performed whole-exome sequencing in two Turkish families with DBQD2. We found a novel and a recurrent XYLT1 mutation in each family. The patients were homozygous for the mutations. Our results further support that XYLT1 is responsible for a major subset of DBQD2.
Similar content being viewed by others
Introduction
Desbuquois dysplasia (DBQD) is a group of skeletal dysplasia with an autosomal recessive inheritance, which belongs to the group 20 of genetic skeletal disorders (‘Dysplasias with multiple joint dislocations’).1 DBQD is characterized by severe prenatal and postnatal growth retardation, joint laxity, short extremities, and progressive scoliosis.2 Its main radiological manifestations are short long tubular bones with metaphyseal splay, a ‘Swedish key’ appearance of the proximal femur, and advanced bone age.2, 3 DBQD is clinically and radiographically heterogeneous. Initially, DBQD was classified into two types based on the presence (DBQD1; MIM 251450) or absence (DBQD2; MIM 615777) of characteristic hand abnormalities. These include accessory ossification distal to the second metacarpal, bifid distal thumb phalanx, delta phalanx, and dislocation of interphalangeal joints.3 Moreover, Kim et al.4 reported an additional DBQD subtype characterized by short metacarpals, elongated proximal and middle phalanges, and very short distal phalanges. The ‘Kim variant’ is currently included in DBDQ1 (MIM 251450).
In DBQD12 and Kim variant,4 CANT1 (MIM 613165) mutations have been identified. Additionally, Furuichi et al.5 reported that one patient with DBQD2 from a consanguineous Turkish family also carried CANT1 mutations, although the negative results were reported by Bui et al.6 after screening for CANT1 in 30 individuals with DBQD2. On the other hand, Bui et al.6 identified five distinct homozygous mutations in the xylosyltransferase 1 gene (XYLT1, MIM 608124) in six consanguineous DBQD2 families. Recently, two other groups reported compound heterozygous XYLT1 mutations and a homozygous mutation in two patients with DBQD2, respectively.7, 8 Xylosyltransferase 1 is an enzyme catalyzing the first step in the biosynthesis of proteoglycan, which is known to play important roles as extracellular matrix organizers and cell signaling mediators.9
In this study, by performing whole-exome sequencing, we identified a novel and a recurrent XYLT1 mutation in three patients with DBQD2 from two Turkish families. Our findings further certified that XYLT1 is responsible for a major subset of DBQD2.
Materials and methods
Patients
Family 1
A pair of sisters was referred to our department for further investigation of skeletal dysplasia, because they had similar severe pre- and postnatal growth retardation. Their healthy Turkish parents were first cousins and had one healthy son and one aborted pregnancy (Supplementary Figure S1a).
Case 1 was a 7-year-old girl who was born as the first child from a 26-year-old mother after full-term gestation by Cesarian section because of fetal distress. Her birth weight was 2600 g. Ultrasound examinations during 6–7 months of gestation showed that she had short extremities, chest wall deformity, and pectus excavatum. Immediately after birth, she was sent to the neonatal intensive care unit and stayed there for a week because of respiratory problems. At her routine follow-up, she was evaluated for her distinct shortness, but all the biochemical, metabolic, and hormonal workout including growth hormone were normal. Her subsequent course of global development was within normal limits, with an exception of her language and social skills. Denver developmental tests at 6 years showed delay of her motor, language, and social developments. Results of physical examination at age 7 years were as follows: weight, 10.6 kg (3rd percentile); height, 76 cm (−9 s.d.); head circumference, 43 cm (−8 s.d.); upper segment, 43 cm; lower segment, 33 cm; and arm span, 70 cm. She presented with flat face, short nose, short neck, narrow chest, minimal scoliosis, increased lumbar lordosis, and hypertrichosis on the back; short limbs, short stubby fingers, bilateral mild hallux valgus, and protruding heels were noted (Figures 1a and b). Her skin over the elbow and knee joints was stiff. Vision, hearing, and cardiovascular, respiratory, and gastrointestinal systems were normal. Abdominal ultrasound showed mild hepatomegaly.
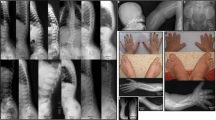
Appearances of the patients. (a, b) Case 1, age 7 years. (a) Flat face, short nose, narrow chest, increased lumbar lordosis, short-limbed short stature, flat foot, and pes valgus. (b) Short stubby fingers. (c) Case 2, age 40 days. Flat face, depressed nasal bridge, short nose, long philtrum, and small hand. (d) Case 3, age 3.5 months. Flat face with prominent eyes, micrognathia, long philtrum, pectus carinatum, and short limbs. A full color version of this figure is available at the Journal of Human Genetics journal online.
Skeletal survey at age 7 years showed exaggerated lumbosacral lordosis, flat proximal femoral epiphyses, short femoral necks with mild prominence of the lesser trochanters (Figures 2a and b), and flat and irregular epiphyses of the distal radius and ulna (Figure 2c). Generalized brachydactyly (particularly brachymetacarpia) and advanced bone age were noted (Figure 2c).
Radiographs of the patients. (a–c) Case 1, age 7 years. (a) Lumbar scoliosis, flat proximal femoral epiphyses, and short femoral necks with mild prominence of the lesser trochanters. (b) Exaggerated lumbosacral lordosis. Vertebral bodies are normal. (c) Flat and irregular epiphyses of the distal radius and ulna, brachydactyly (particularly brachymetacarpia), and advanced bone age. (d) Case 2, age 40 days. Babygram showing short and broad long tubular bones, short femoral necks with prominent lesser trochanters. (e–g) Case 2, age 2 years 3 months. (e) Mild platyspodyly with notching of the posterior endplates of the lumbar spine. (f) Prominent lesser trochanters, epiphyseal dysplasia, and broad and short long tubular bones. (g) Epiphyseal dysplasia of the long tubular bones, elbow dislocation, advanced bone age, and brachymetacarpia (particularly the first metacarpal). (h) Case 3, age 6 months. Short and broad long tubular bones and short femoral neck with prominent lesser trochanters.
Case 2 was the younger sister of Case 1. She was also found to have short extremities and intrauterine growth retardation, pectus excavatum, and hepatomegaly by ultrasound examination at 6 months of gestation. She was born at 38 weeks of gestation by Cesarian section. Her birth weight was 1800 g. On physical examination at 40 days, her weight was 2800 g (<3rd percentiles), height 37 cm (−9 s.d.), head circumference 34 cm (<−3 s.d.), upper segment 26 cm, and lower segment 11 cm. She showed a distinct shortness with relative macrocephaly and short limbs, short neck, short thorax, and distended abdomen. She presented with flat face, high frontal area, prominent eyes, depressed nasal bridge, short nose, anteverted nostrils, and smooth long philtrum (Figure 1c). Examinations of cardiopulmonary, liver, and nervous systems were normal.
A babygram at 40 days of age showed ovoid vertebral bodies, short and broad long tubular bones, and short femoral necks with mild prominence of the lesser trochanters (Figure 2d). Follow-up skeletal survey at age 2 years and 3 months revealed spondylar dysplasia, prominent lesser trochanters, epiphyseal dysplasia, short and broad long tubular bones, elbow dislocation, and advanced carpal bone age (Figures 2e–g).
Family 2
Case 3 was a 4-month-old girl presented with severe shortness of prenatal onset. She was born as a first child from young, healthy parents (Supplementary Figure S1b). Heights of the father and mother were 163 and 153 cm, respectively. Parents were not consanguineous but came from neighboring villages. Ultrasound examination at 19th week of gestation detected intrauterine growth retardation with short femur. She was born at 36 weeks of gestation by Cesarian section and was entubated because of respiratory distress. Her birth weight was 1700 g (<3rd percentile), birth length 36 cm (−7 s.d.), and occipito-frontal circumference 31 cm (−3 s.d.). Disproportional shortness and cleft palate were noted. At 4 months of age, her weight was 2100 g (<3rd percentile), length 41 cm (−9 s.d.), occipito-frontal circumference 34.5 cm (−6.5 s.d.), upper segment 26 cm, and lower segment 15 cm. She had macrocephaly, flat round face, hypertelorism, prominent eyes, blue sclerae, depressed nasal bridge, short stubby nose, antevert nostrils, microretrognaty, cleft palate, narrow thorax, pectus carinatum, distended abdomen, and micromelic extremities (Figure 1d).
A babygram at 6 months showed short and broad long tubular bones, short femoral neck with prominent lesser trochanters, Swedish key like appearance, and advanced bone age (Figure 2h).
Whole-exome sequencing and variant calling
The study protocol was approved by the ethical committee of RIKEN and participating institutions. Peripheral blood was obtained from the family members after the informed consent. Genomic DNA was extracted from the blood using QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany). DNAs concentration was measured using a Qubit V.2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA). Whole-exome sequencing was performed as previously described.10, 11 Briefly, DNA (3 μg) was sheared with S2 Focused-ultrasonicator (Covaris, Wobum, MA, USA) and processed by SureSelectXT Human All Exon V5 (Agilent Technologies, Santa Clara, CA, USA). Captured DNA was sequenced using HiSeq 2000 (Illumina, San Diego, CA, USA) with 101 bp pair-end reads with seven indices. Image analysis and base calling were performed using HCS, RTA and CASAVA software (Illumina). Reads were mapped to the reference human genome (hg19) by Novoalign-3.02.04. Aligned reads were processed by Picard to remove PCR duplicates. Variants were called by GATK v2.7-4 following GATK Best Practice Workflow v312 and annotated by ANNOVAR.13
PCR and Sanger sequencing
Sanger sequencing was performed to confirm the mutation identified by the whole-exome sequencing. Fragments including the mutations identified by whole-exome sequencing were amplified by PCR and sequenced both strands. The primers were 5′-CTAATTCGGGTCCAGCAGAG-3′ and 5′-CGTCAGGCTCATCGTAGACA-3′ (for c.1792delC); and 5′-GCAAATGCAAAGGTCCTGAGG-3′ and 5′-GCTGGTGCCAAGCCTTTCTC-3′ (for c.1290-2A>C). A 3730 DNA analyzer (Life Technologies) was used for the Sanger sequencing. Sequencher V.4.7 (Gene Codes, Ann Arbor, MI, USA) and Genetyx (Genetyx Inc., Tokyo, Japan) were used for aligning sequencing chromatographs to reference sequences.
Evaluation of the mutation identified in XYLT1
The mutations were evaluated by using five databases, dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/), 1000 genomes (http://www.1000genomes.org/), ExAC (http://exac.broadinstitute.org/), ESP6500 (http://evs.gs.washington.edu/EVS/), and Human Gene Mutation Database (https://portal.biobase-international.com/hgmd/pro/start.php). The function of splice-site mutations was evaluated by three online prediction programs, Human Splice Finder (http://www.umd.be/HSF/), NNSPLICE (http://www.fruitfly.org/seq_tools/splice.html), and SplicePort (http://spliceport.cbcb.umd.edu/). Homozygosity mapping was performed by Homozygosity Mapper as previously described14 on whole-exome sequencing data.
Results and discussion
We performed whole-exome sequencing in three patients with DBQD2 from two Turkish families and obtained 1.5, 1.8, and 1.6 Gb sequences, respectively. The sequences were successfully mapped to all human RefSeq. At least 95.8% of all coding regions were covered in a depth of 10 reads (Supplementary Table S1).
We identified a homozygous 1-bp deletion (NM_ 022166.3, c.1792delC) in exon 9 of XYLT1 in both patients of Family 1 (Cases 1 and 2). The deletion was not annotated in any available databases, including dbSNP, 1000 genomes, ExAC, ESP6500, and Human Gene Mutation Database. Homozygosity mapping using the whole-exome sequencing data showed that XYLT1 resided in a 4.0 Mb homozygous region, which was shared by the two sibling patients. We also identified a homozygous splice-site mutation (NM_ 022166.3, c.1290-2A>C) in the splice acceptor site of intron 5 of XYLT1 in the patient from Family 2 (Case 3). Although the mutation has been deposited in Human Gene Mutation Database (CS142006), it was not annotated in dbSNP, 1000 genomes, ExAC, and ESP6500. By Sanger sequencing, we confirmed the homozygous deletion in Cases 1 and 2 and the homozygous splice-site mutation in Case 3 (Supplementary Figure S2). Both mutations were heterozygous in their parents. The deletion in Cases 1 and 2 was absent in their normal sibling.
XYLT1 is composed of 12 exons and encodes a protein of 959 amino acids. c.1792delC causes a premature stop codon (p.R598Afs*7) in exon 9, which is considered to induce nonsense-mediated mRNA decay. c.1290-2A>C is located at the junction between intron 5 and exon 6, and predicted to destroy the acceptor splice sites by several online programs, including Human Splice Finder, NNSPLICE, and SplicePort.
Until now, seven distinct homozygous mutations in XYLT1 have been identified in eight consanguineous DBQD2 families:6, 8, 15 Two are truncating mutations (p.P93Afs*69 and p.R147*), three are missense mutations (p.R598C, p.R481W, and p p.R551C), and two are splicing mutations (c.1290-2A>C and c.1588-3C>T) (Supplementary Table S2). c.1290-2A>C was recurrent and common in Turkish. In addition to these homozygous mutations, compound heterozygous XYLT1 mutations were reported recently in a DBQD2 patient from a non-consanguineous family7 (Supplementary Table S2). Including the families of the present study, a total of 11 families with DBQD2 carrying XYLT1 mutations came from six different ethnic groups (Turkish, Tunisian, Mauritian, Belgian, Polish, and Brazilian), suggesting that the causal role of XYLT1 in DBQD2 is independent of ethnic origin.
Three of seven cases with XYLT1 mutations reported by Bui et al. showed brachymetacarpia,6 a phenotype overlapping with DBQD1, Kim variant.4 Brachymetacarpia was also observed in two DBQD2 cases with XYLT1 mutations in our study and another two XYLT1-associated DBQD2 cases reported recently.7, 8 All the three cases in our study presented microcephaly, which is consistent with the previous reports,6, 7, 15 although one case reported recently showed macrocephaly.8
DBQD2 has overlapping manifestations with many skeletal dysplasias other than DBQD1, including autosomal dominant form of Larsen syndrome (MIM 150250), spondylo-epiphyseal dysplasia with congenital joint dislocations (MIM 143095), diastrophic dysplasia (MIM 222600), and chondrodysplasia with joint dislocations, GPAPP type (MIM 614078). Therefore, differential diagnosis based on clinical features is sometimes difficult; we have previously experienced a case with intermediate phenotype of DBQD, diastrophic dysplasia and recessive form of multiple epiphyseal dysplasia, which was found to have DTDST (MIM 606718) mutations.16 We screened the known causal genes for these diseases in our data set, including CANT1,2 DTDST,16 FLNB (MIM 603381),17 CHST3 (MIM 603799),18 SLC26A2 (MIM 606718),19 and IMPAD1 (MIM 614010).20 No mutations were found in these genes. The exome-based approach has an advantage over the gene-by-gene analysis in the diagnosis of diseases with wide heterogeneity and phenotypic variations like DBQD.
References
Bonafe, L., Cormier-Daire, V., Hall, C., Lachman, R., Mortier, G., Mundlos, S. et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am. J. Med. Genet. A 167, 2869–2892 (2015).
Huber, C., Oulès, B., Bertoli, M., Chami, M., Fradin, M., Alanay, Y. et al. Identification of CANT1 mutations in Desbuquois dysplasia. Am. J. Hum. Genet. 85, 706–710 (2009).
Faivre, L., Cormier-Daire, V., Eliott, A. M., Field, F., Munnich, A., Maroteaux, P. et al. Desbuquois dysplasia, a reevaluation with abnormal and ‘normal’ hands: radiographic manifestations. Am. J. Med. Genet. A 124A, 48–53 (2004).
Kim, O., Nishimura, G., Song, H., Matsui, Y., Sakazume, S., Yamada, M. et al. A variant of Desbuquois dysplasia characterized by advanced carpal bone age, short metacarpals, and elongated phalanges : report of seven cases. Am. J. Med. Genet. A 152A, 875–885 (2010).
Furuichi, T., Dai, J., Cho, T., Sakazume, S., Ikema, M., Matsui, Y. et al. CANT1 mutation is also responsible for Desbuquois dysplasia, type 2 and Kim variant. J. Med. Genet. 48, 32–37 (2011).
Bui, C., Huber, C., Tuysuz, B., Alanay, Y., Bole-Feysot, C., Leroy, J. G. et al. XYLT1 mutations in desbuquois dysplasia type 2. Am. J. Hum. Genet. 94, 405–414 (2014).
Jamsheer, A., Olech, E. M., Kozłowski, K., Niedziela, M., Sowińska-Seidler, A., Obara-Moszyńska, M. et al. Exome sequencing reveals two novel compound heterozygous XYLT1 mutations in a Polish patient with Desbuquois dysplasia type 2 and growth hormone deficiency. J. Hum. Genet. 61, 577–583 (2016).
Silveira, C., Leal, G. F. & Cavalcanti, D. P. Desbuquois dysplasia type II in a patient with a homozygous mutation in XYLT1 and new unusual findings. Am. J. Med. Genet. A 170, 3043–3047 (2016).
Prydz, K. & Dalen, K. T. Synthesis and sorting of proteoglycans. J. Cell Sci. 113, 193–205 (2000).
Nakajima, M., Mizumoto, S., Miyake, N., Kogawa, R., Iida, A., Ito, H. et al. Mutations in B3GALT6, which encodes a glycosaminoglycan linker region enzyme, cause a spectrum of skeletal and connective tissue disorders. Am. J. Hum. Genet. 92, 927–934 (2013).
Nakajima, J., Okamoto, N., Tohyama, J., Kato, M., Arai, H. & Funahashi, O. De novo EEF1A2 mutations in patients with characteristic facial features, intellectual disability, autistic behaviors and epilepsy. Clin. Genet. 87, 356–361 (2015).
Mckenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A. et al. The Genome Analysis Toolkit : A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR : functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, 1–7 (2010).
Miyatake, S., Tada, H., Moriya, S., Takanashi, J., Hirano, Y., Hayashi, M. et al. Atypical giant axonal neuropathy arising from a homozygous mutation by uniparental isodisomy. Clin. Genet. 87, 395–397 (2015).
Schreml, J., Durmaz, B., Cogulu, O., Keupp, K., Beleggia, F., Pohl, E. et al. The missing ‘link’: an autosomal recessive short stature syndrome caused by a hypofunctional XYLT1 mutation. Hum. Genet. 133, 29–39 (2014).
Miyake, A., Nishimura, G., Futami, T., Ohashi, H., Chiba, K., Toyama, Y. et al. A compound heterozygote of novel and recurrent DTDST mutations results in a novel intermediate phenotype of Desbuquois dysplasia, diastrophic dysplasia, and recessive form of multiple epiphyseal dysplasia. J. Hum. Genet. 53, 764–768 (2008).
Zhang, D., Herring, J. A., Swaney, S. S., McClendon, T. B., Gao, X., Browne, R. H. et al. Mutations responsible for Larsen syndrome cluster in the FLNB protein. J. Med. Genet. 43, e24 (2006).
Unger, S., Lausch, E., Rossi, A., Mégarbané, A., Sillence, D., Alcausin, M. et al. Phenotypic features of carbohydrate sulfotransferase 3 (CHST3) deficiency in 24 patients: congenital dislocations and vertebral changes as principal diagnostic features. Am. J. Med. Genet. A 152A, 2543–2549 (2010).
Hästbacka, J., de la Chapelle, A., Mahtani, M. M., Clines, G., Reeve-Daly, M. P., Daly, M. et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell 78, 1073–1087 (1994).
Vissers, L. E., Lausch, E., Unger, S., Campos-Xavier, A. B., Gilissen, C., Rossi, A. et al. Chondrodysplasia and abnormal joint development associated with mutations in IMPAD1, encoding the Golgi-resident nucleotide phosphatase, gPAPP. Am. J. Hum. Genet 88, 608–615 (2011).
Acknowledgements
We thank the patients and their families for their help to the study. We also thank N Atsumi for checking English. This study is supported by research grants from the Japan Agency For Medical Research and Development (AMED) (contract no. 14525125).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Guo, L., Elcioglu, N., Iida, A. et al. Novel and recurrent XYLT1 mutations in two Turkish families with Desbuquois dysplasia, type 2. J Hum Genet 62, 447–451 (2017). https://doi.org/10.1038/jhg.2016.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.143
This article is cited by
-
Novel compound heterozygous variants in XYLT1 gene caused Desbuquois dysplasia type 2 in an aborted fetus: a case report
BMC Pediatrics (2022)
-
Cloning, expression and enzyme activity delineation of two novel CANT1 mutations: the disappearance of dimerization may indicate the change of protein conformation and even function
Orphanet Journal of Rare Diseases (2020)
-
Recapitulating the human segmentation clock with pluripotent stem cells
Nature (2020)
-
The clinical and mutational spectrum of B3GAT3 linkeropathy: two case reports and literature review
Orphanet Journal of Rare Diseases (2019)
-
Dysosteosclerosis is also caused by TNFRSF11A mutation
Journal of Human Genetics (2018)