Abstract
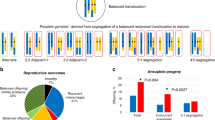
Forty-six reciprocal and six Robertsonian translocation carrier couples who experienced recurrent pregnancy loss underwent fluorescence in situ hybridization-based preimplantation genetic diagnosis (PGD) for the presence of the two translocated chromosomes. Out of 52 couples, 17 (33%) were undergoing infertility treatment. In total, 239 PGD cycles as oocyte retrieval (OR) were applied. The transferrable rate of negatively diagnosed embryos at the cleavage stage was 26.3%; 71 embryos were transferred as single blastocysts. The clinical pregnancy rate per transfer was 60.6%. We obtained 41 healthy live births with 3 incidences of miscarriage (7.0%). The average cumulative live birth rate was 76.9% during 4.6 OR cycles using a mild ovarian stimulation strategy. The outcomes were classified into four groups based on carrier gender and maternal age (young (<38 years) or advanced). PGD was performed for 52 couples of which the average number of OR cycles was 4.1, 2.1, 6.7 and 4.5 in young female and male carriers and female and male carriers of advanced age; the live birth rate for a primiparity was 77.8, 72.7, 66.7 and 50.0% in those groups. These results suggest that the final live birth rate might be influenced by maternal age regardless of the gender of the carrier.
Similar content being viewed by others
Introduction
Carriers of autosomal reciprocal translocations and Robertsonian translocations often have the opportunity to reproduce, however, these couples are known to be at increased risk for reproductive difficulties such as recurrent pregnancy loss (RPL) or phenotypically abnormal offspring owing to their production of genetically unbalanced gametes. In these carriers, the segmental affinities between the normal and translocated chromosomal regions result in quadrivalent rather than bivalent pairing at meiosis with five potential modes of segregation, and this phenomenon produces unbalanced rearrangements at high incidence.1
The first establishment of fluorescence in situ hybridization (FISH)-based preimplantation genetic diagnosis (PGD) for translocation carriers was reported about 10 years after the first PGD was described by Handyside et al.,2, 3, 4, 5 and this technique has been utilized in clinical trials for patients suffering from reproductive difficulties.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Among these 15 studies, the reproductive outcomes of a total of 4700 carriers have been described with the pregnancy rate per embryo transfer at 22–52%, live birth rate per patient at 28–44% (average 34%) and pregnancy loss (number of spontaneous abortions per clinical pregnancy) at 0–18%.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20
Female carriers who maintain normal ovarian function equivalent to that of females with normal karyotypes are able to sustain controlled ovarian stimulation with gonadotropin.20 Most reports have described that the ovarian response to controlled ovarian stimulation was not impaired by translocation status, suggesting that no differences of the segregation pattern (alternate vs unbalanced) in the PGD results or of the live birth rate between the gender and carrier groups existed.12, 13, 14, 15, 18, 20, 21, 22 The mechanism governing whether autosomal translocation carriers experience premature ovarian failure or can maintain normal ovarian response has not yet been defined; however, most autosomal translocation carriers retain their fecundity. Therefore, all institutes that practice PGD advocate the application of standard ovarian stimulation to such carriers, representing the controlled ovarian hyperstimulation protocol utilized for regular infertility treatment in each facility. Notably, however, the reproductive outcome of RPL couples utilizing PGD is less efficient than that obtained following natural pregnancy.23, 24 To potentially improve these outcomes, we established an original in vitro fertilization (IVF) protocol featuring dose reduction of gonadotropin for ovarian stimulation, which was able to decrease the incidence of ovarian hyperstimulation syndrome and multiple pregnancies.25, 26, 27 In addition, our protocol was expected to improve reproductive outcome even in advanced maternal age couples with ovulation disorder.28
In this paper, we report the clinical outcomes of FISH-based PGD using mild ovarian stimulation and single blastocyst transfer policy for 52 couples, in which one of the partners was an autosomal reciprocal translocation or Robertsonian translocation carrier. We retrospectively evaluated the live birth rate and the chromosomal imbalances of embryos derived from these RPL couples and determined how maternal age affected the reproductive outcome.
Materials and methods
Patients
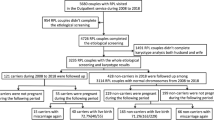
There are strict guidelines for the performance of PGD in Japan established by the Japan Society of Obstetrics and Gynecology (JSOG). These state that patients should be given genetic counseling by a certified specialist in Medical Genetics or a genetic counselor at a clinical research center as well as from a third party institution as a second opinion, and that written consent should be required in the application. For situations involving habitual spontaneous abortion or high-risk pregnancies with serious genetic disorders, all individual cases should be evaluated by consecutive ethical committees at the supervising clinical research center and by JSOG. All couples who underwent PGD in this study were required to satisfy the following two conditions: (i) one partner was a carrier of a chromosomal abnormality; and (ii) a history of RPL, defined as two or more previous miscarriages that were attributed to either partner’s chromosomal abnormality, and obstetric history, or serious genetic disorders with a prospectively high-risk pregnancy. Genetic counseling was administered to 90 PGD candidate couples who had an abnormal karyotype in either partner and written consent was obtained for this PGD clinical study with provision of an explanation of the benefits and drawbacks by our certified specialist in Medical Genetics and genetic counselor. All 90 cases were approved by the Institutional Review Board of the Kato Ladies Clinic on the first review; out of 90 couples, 88 were eventually accredited for PGD by the JSOG ethics committee during the second stage of review, and 2 cases were rejected as they were considered to be outside of the indication.
This clinical study involved the provision of PGD for the 52 accredited couples who have a reciprocal translocation (n=46) or Robertsonian translocation (n=6) in either partner, between January 2007 and December 2013 at the Kato Ladies Clinic. Of the 52 couples in the RPL group, 17 infertile patients (6 oligozoospermia, 11 fallopian tubal factor or premature ovarian insufficiency) were included. The maternal age of the 33 female carriers and 19 normal karyotype females whose husbands were carriers was 36.2 and 37.2 years, respectively; all of the 52 couples had previously experienced two or more miscarriages (range: 2–7) before 20 weeks. Only three of the couples had experience of delivery representing one case of a healthy child, one case of stillbirth and one case of neonatal death with structural imbalance (Table 1). PGD was performed for the 52 couples with RPL, which included 33% (17/52) infertile patients.
Minimal stimulation and natural cycle IVF protocol
The controlled ovarian stimulation protocol is commonly used for infertility treatments to obtain as many oocytes as possible, and is also utilized for PGD protocols; however, we have established alternate unstimulated and mild ovarian stimulation cycles for the assisted reproductive technology protocol that we apply in daily clinical practice. In this study, the unstimulated and minimal stimulation IVF protocols were performed as described previously.25, 26, 27 In brief, a clomiphene citrate-based minimal stimulation protocol was used in the majority of cycles (87.5%), whereas unstimulated natural (or using clomiphene citrate without gonadotropin) IVF was performed for the remaining cycles (12.5%). Clomiphene citrate (50–100 mg per day) was administrated orally with an extended regimen from cycle day 3 until the day before inducing final oocyte maturation. Human menopausal gonadotropin or recombinant follicle-stimulating hormone was provided in the form of injections (50–150 IU/every other day) or human menopausal gonadotropin nasal spray for the clomiphene citrate-based stimulation protocols to obtain several matured follicles. Monitoring involving ultrasound scans and hormonal profiles (estradiol, luteinizing hormone and progesterone) was usually started on day 8 and continued every other day until the triggering day. Ovulation triggering was performed with a gonadotropin-releasing hormone agonist, busereline (Suprecur, 600mg, Sanoffi K.K. Co., Ltd, Tokyo, Japan) administered in nasal spray form. In the natural cycle IVF protocol, the only pharmaceutical intervention consisted of inducing the final oocyte maturation with a gonadotropin-releasing hormone agonist. Monitoring as described above was started in the morning of day 10 and/or 12 according to the patient’s menstruation cycle length. When the leading follicle reached 18 mm with a concomitant estradiol level >250 pg ml−1, oocyte retrieval (OR) was scheduled. OR was usually conducted 32–35 h after triggering, but it was also performed 20–30 h after triggering for some patients when the start of the LH surge was detected.
OR, IVF and blastocyst culture
OR was practiced without anesthesia using an extra-thin 21 G needle (Kitazato BioPharma Co., Ltd., Fuji City, Japan). Conventional insemination was performed approximately 3 h after OR and intracytoplasmic sperm injection was performed as the insemination method in the presence of moderate or severe male factor infertility. Normally fertilized zygotes with two pro-nuclei, which were assessed 16–20 h after insemination, were cultured individually with Quinn’s Advantage Protein Plus cleavage medium (Origio, Malov, Denmark) from day 1 to 3. All biopsied embryos at the cleavage stage were transferred to Quinn’s Advantage Protein Plus blastocyst medium from day 4 to 6.
Zona breaching, embryo biopsy, fixation and FISH
Embryo biopsy was performed on the night of day 2 after OR to the morning of day 3, mechanical zona cutting was performed as previously described by Verlinsky with some modifications.29, 30 A 2-blastomere biopsy strategy was practiced for 5–10-cell stage embryos using aspiration by a thin pipette (Kitazato BioPharma Co., Ltd., Japan). Blastomeres were fixed using the Carnoy fixative method that was described by Velilla et al.31, 32, 33 with some modifications. We chose to use the subtelomeric probes method that was described by Scriven et al.5 with some modifications: in the case of two-way translocation, two differentially labeled subtelomeric probes that were distal to the breakpoints on the translocated segments and another two subtelomeric probes that were on the opposite centric segment from the breakpoint were applied for chromosome enumeration. The subtelomeric FISH probes were purchased from GSP Lab., Inc. (Abnova, Taipei, Taiwan) and the recommended procedure was followed with some modifications. In total, four FISH probes were used to analyze the numbers of each of the two segments and two chromosomes of the two-way translocations, and FISH results were assigned as being consistent with alternate, 2:2 adjacent-1, 2:2 adjacent-2, 3:1 or 4:0 disjunction at meiosis I using the FISH scoring criteria described by Scriven et al.5 and Munne et al.6
Single vitrified-warmed blastocyst transfer
In all cases, embryos were cultured to blastocysts after being biopsied. Blastocysts that showed negative FISH results and were morphologically normal were vitrified electively for subsequent use in the transfer cycle.
Vitrification using the Cryotop vitrification kit (Kitazato BioPharma Co., Ltd.) has been described previously34 and frozen-thawed embryo transfers were performed in spontaneous natural cycles on day 5 after ovulation was confined. Embryo transfer procedures were performed under vaginal ultrasound guidance using a specially designed soft catheter (Kitazato BioPharma Co., Ltd.) by placing a single embryo in a minimal volume into the mid-uterine cavity.35
Results
Out of 52 couples completing PGD who had a history of RPL, 33 female carriers and 19 females with normal karyotype whose husbands were translocation carriers had average maternal ages of 36.2 and 37.2 years, respectively (Table 1).
Out of 239 OR cycles using unstimulated natural cycles and mild ovarian stimulation, 174 were performed in the female carrier group and 65 in the male carrier group; the average number of oocytes retrieved was 2.4±2.1(s.d.; range: 1–16) and 2.9±2.0 (1–10), respectively. No significant differences were identified in factors including the cancellation rate, median number of cumulus oocyte complexes in successful OR cycles, 2 pronucleus (2PNs) oocytes, cleavage embryo formation rates, transferrable rate, pregnancy and live birth rate, and the miscarriage rate between female and male carrier groups (Table 2).
We also compared the reproductive outcome for obtaining primiparity between the four groups within our cohort that consisted of carrier gender and maternal age (young: <38 years; advanced: ⩾38) as shown in Table 3. A higher cumulative live birth rate was seen in the young maternal age group: 77.8 and 72.7% in young female carriers and young normal karyotype females whose husbands were in the carrier group; in the advanced maternal age group, the rate was 66.7 and 50.0% in the advanced maternal age carrier and advanced maternal age normal karyotype groups. The distribution of the number of OR cycles needed to obtain primiparity is shown in Table 3; the average number of OR cycles in the successful group was 3.8 and 1.6 in the young female carrier and young normal karyotype female group, respectively, and 7.4 and 3.8 in the advanced maternal age carrier and advanced normal karyotype female group, respectively. In general, Robertsonian translocation carrier couples have a high risk of abortion and delivery of unbalanced karyotype with full aneuploidy for chromosome 13 or 21; however, these couples show better reproductive outcome. In the present study, six Robertsonian translocation couples were included among the 52 carrier couples and there was no Robertsonian translocation carrier couple in the young male carrier couple group (Table 3). The cumulative live birth rate in the Robertsonian carrier couple group was 66.7% (4/6), which was considered to be the same level as compared with autosomal reciprocal translocation group.
In general, the IVF success rate increases with the numbers of OR cycles until reaching a plateau. Our cumulative success data were the most effective; however, the number of OR cycles was also the highest, which was 4.6 times on average and exceeded the number obtained by another institute by approximately twofold.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Young females whose husbands were in the young carrier group required the fewest PGD cycles: 1.6, compared with 7.4 for the advanced age female carrier group.
In addition, we presented PGD outcome measures for patients with reciprocal translocations from both single centers15, 16 and a multiple center, which were collected by the European Society of Human Reproduction and Embryology (ESHRE) PGD Consortium19 along with data from the reproductive immunologist group from natural pregnancy23, 36 (Table 4). Overall, 52 translocation carrier couples with RPL in the present study achieved an average 76.9% (40/52) cumulative live birth rate, which included four couples trying for their second child with PGD. This rate is higher than that obtained in previous PGD reports.
Discussion
There are two primary choices of RPL treatments for couples in which either partner is a translocation carrier: natural pregnancy with concomitant reproductive immunological management and IVF with PGD; the issue of which method should be utilized preferentially is not clear. To our knowledge, based on results from reports of PGD6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 and the conservative strategy using natural pregnancy,23, 24, 36 we consider that the natural pregnancy strategy seems to have better prognosis in young reciprocal translocation carrier couples with RPL. It is difficult to understand these results; however, the patient background, biopsy invasiveness and diagnostic accuracy might influence the final outcome. Consistent with this, Ikuma et al.24 reported a 68% cumulative live birth rate using PGD in a 31-year maternal age RPL couple group in a randomized control study, with the conclusion that PGD might perform similarly with respect to the live birth rate compared with natural pregnancy while preventing further miscarriages in the young RPL couple group. However, there are very few clinical reports that are concerned about the reproductive outcome of advanced maternal age couples wherein either partner is a translocation carrier with RPL. Keymolen et al.15 reported that the cumulative pregnancy rate (number of pregnancies/couple) in an advanced age (>35 years) group was 22% (4/18) and 5% (1/20) in the female and male carrier groups, respectively. In addition, another report described a cumulative pregnancy rate of 8% (1/12) in advanced maternal age (>38 year) couples.14 In the present study, we achieved 67 and 50% cumulative live births in the advanced maternal age (⩾38 year) female and male carrier groups using PGD.
It is known that maternal age strongly influences IVF outcomes, and our results suggested that it also affects reproductive outcome in the chromosomal structural abnormality carrier couples. In the young maternal age group (⩽37 years), the average number of PGD cycles as OR cycles to obtain about a 70% live birth rate was 4.1 and 2.1 in female carriers and normal karyotype females whose husbands were in the carrier group, respectively. Furthermore, in the advanced maternal aged group (⩾38 years), 6.7 PGD cycles were required in the female carrier group, and 4.5 cycles were needed to obtain 50% successful rate in women with a normal karyotype whose husbands were in the carrier group (Table 3).
However, these results did not allow a conclusion to be drawn regarding the underlying mechanism of how translocation influences the prognosis of reproductive outcome. Some studies indicated some trend for the relationship between the type of chromosomal abnormality or carrier gender and reproductive outcome. It has been established that young carrier Robertsonian translocation couples have good prognosis not only without PGD36, 37 but also with PGD.19 It was revealed that young male Robertsonian translocation carriers showed statistically higher17 or trends toward higher12, 13, 15, 20 reproductive outcomes than did young female carriers in the PGD clinical study. However, no young male Robertsonian translocation carriers were enrolled in the present study, and the cumulative live birth rate of all Robertsonian translocation carrier couples was 66.7% (4/6), which was the same result as that of autosomal translocation couples. Furthermore, Keymolen et al.15 suggested that the younger male reciprocal translocation carrier group showed the best prognosis of live birth rate per couple and the worst rate was obtained in the advanced age male carrier couple group. In addition, the younger female group performed better than the advanced age female group, but this difference was small. The same trend was observed in the present study, wherein the most effective average number of PGD cycles as oocyte retrieval was 2.1 in young normal karyotype females whose husbands were in the young carrier (avg. 36.6 years) group. The number of IVF/PGD cycles available or required represents one of the relevant factors, as Scriven et al.16 described that carrier couples would be afforded a 50% chance of successful live birth following three cycles of PGD with an ovarian hyperstimulation protocol. Comparatively, in the present study, 4.6 PGD cycles with mild ovarian stimulation were sufficient to obtain a 77% chance of successful live birth (Table 4).
The first report of the existence of an interchromosomal effect was written by Lejeune in 1963;38 this has been a controversial topic ever since. Some patients exhibiting a higher than average rate of chromosomal abnormality have been discovered among the translocation carrier population;39, 40, 41, 42, 43, 44 however, we consider that whether this phenomenon was caused by the concurrent translocation remains unclear. In the present study, FISH was only applied for examination of the two translocated chromosomes during the process of PGD, whereas the transferred embryos subsequently had equivalent aneuploidy risk as compared with the general embryos used in non-PGD IVF cycles. However, we obtained relatively favorable reproductive outcomes in the translocation carrier group with 37 years average maternal age, and the clinical pregnancy rate (fetal heartbeat positive; implantation rate) per single blastocyst transfer and miscarriage rate per clinical pregnancy were 62.0% and 7.0%, respectively. Out of three miscarriage cases, chromosomal analysis of the abortus was performed for two cases, identifying 45,X and trisomy 21. In comparison, in the general sub-fertile couple group at 35–37 years of maternal age treated in our IVF center, the clinical results for these rates were 45.0% and 17.5%,35 respectively. Furthermore, in the case of natural conception with a maternal age of 30–40 years, the rate of early pregnancy loss was 8–15%,45, 46, 47, 48 and 57–75% of chromosomal abnormality was found in the product of conception.49, 50, 51 Thus, we considered that interchromosomal effect might not substantively influence meiosis in our translocation carrier patient group.
In the present study, we achieved a 76.9% cumulative live birth rate with 4.6 oocyte retrieval cycles using FISH-based PGD for 52 couples with a history of RPL who had 46 reciprocal and 6 Robertsonian translocations. The average chromosomal abnormality rate of the embryos was 73.7%. The IVF procedure was performed with our original mild ovarian stimulation protocol and single blastocyst transfer policy. Using our IVF protocol for general infertile patients, the expected cumulative live birth rate is 77, 70 and 45% in <35, 36 and 39 year maternal age groups during five OR cycles.28 In the present study, translocation carrier couples showed the same reproductive outcome compared with the general infertility population by excluding the unbalanced rearrangement embryos for transfer using PGD. However, considering that the number of embryos was reduced to only 1/5 to 1/3, because the average transferrable rate of reciprocal translocation carrier couples was 20–30% on a global basis6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 because most embryos were eliminated by PGD as being affected with a chromosomal abnormality related to translocated chromosomes, this RPL couple group might have a better ability for reproduction compared with general infertility patients. In particular, the best group, which showed a 72.7% live birth rate for primiparity with the fewest average number of OR cycles (2.1 cycles), was that of young normal karyotype females whose husbands were in the young carrier group. These young couples might have the chance to obtain live birth with natural pregnancy if they were not adverse to a high abortion risk, which is associated with the reciprocal translocation; conversely, in the case of advanced age couples, the worthful way to achieve the goal of a live birth might be to use an IVF/PGD protocol if the higher cost, time and effort required for IVF and the invasiveness of the biopsy are fully acceptable.
More studies are needed to confirm these results and to interpret the results with respect to underlying differences in the mechanisms of meiosis in spermatocytes and primary oocytes. Overall, we consider that the accumulation of these detailed reproductive outcomes of translocation carriers with or without PGD will likely contribute to an extensive discussion of genetic counseling.
References
Gardner, R. J. M., Sutherland, G. R. & Shaffer, L. G. Chromosome Abnormalities and Genetic Counselling, 4th edition, pp67, 99–111 (Oxford University Press, New York, NY, USA, 2012).
Handyside, A. H., Kontogianni, E. H., Hardy, K. & Winston, R. M. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 344, 768–770 (1990).
Cassel, M. J., Munne, S., Fung, J. & Weier, H. U. Carrier-specific breakpoint-spanning DNA probes: an approach to preimplantation genetic diagnosis in interphase cells. Hum. Reprod. 12, 2019–2027 (1997).
Pierce, K. E., Fitzgerald, L. M., Seibel, M. M. & Zilberstein, M. Preimplantation genetic diagnosis of chromosome balance in embryos from a patient with a balanced reciprocal translocation. Mol. Hum. Reprod. 4, 167–172 (1998).
Scriven, P. N., Handyside, A. H. & Ogilvie, C. M. Chromosome translocations: segregation modes and strategies for preimplantation genetic diagnosis. Prenat. Diagn. 18, 1437–1449 (1998).
Munne, S., Sandalinas, M., Escudero, T., Fung, J., Gianaroli, L. & Cohen, J. Outcome of preimplantation genetic diagnosis of translocations. Fertil. Steril. 73, 1209–1218 (2000).
Kyu Lim, C., Hyun Jun, J., Mi Min, D., Lee, H. S., Young Kim, J., Koong, M. K. et al. Efficacy and clinical outcome of preimplantation genetic diagnosis using FISH for couples of reciprocal and Robertsonian translocations: the Korean experience. Prenat. Diagn. 24, 556–561 (2004).
Verlinsky, Y., Tur-Kaspa, I., Cieslak, J., Bernal, A., Morris, R., Taranissi, M. et al. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod. Biomed. Online 11, 219–225 (2005).
Otani, T., Roche, M., Mizuike, M., Colls, P., Escudero, T. & Munné, S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod. Biomed. Online 13, 869–874 (2006).
Grifo, J., Talebian, S., Keegan, D., Krey, L., Adler, A. & Berkeley, A. Ten-year experience with preimplantation genetic diagnosis (PGD) at the New York University School of Medicine Fertility Center. Fertil. Steril. 88, 978–981 (2007).
Fischer, J., Colls, P., Escudero, T. & Munne, S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil. Steril. 94, 283–289 (2010).
Ko, D. S., Cho, J. W., Park, S. Y., Kim, J. Y., Koong, M. K., Song, I. O. et al. Clinical outcomes of preimplantation genetic diagnosis (PGD) and analysis of meiotic segregation modes in reciprocal translocation carriers. Am. J. Med. Genet. A 152A, 1428–1433 (2010).
Lledo, B., Ortiz, J. A., Morales, R., Ten, J., de la Fuente, P. E., García-Ochoa, C. et al. The paternal effect of chromosome translocation carriers observed from meiotic segregation in embryos. Hum. Reprod. 25, 1843–1848 (2010).
Xanthopoulou, L., Mantzouratou, A., Mania, A., Ghevaria, H., Ghebo, C., Serhal, P. et al. When is old too old for preimplantation genetic diagnosis for reciprocal translocations? Prenat. Diagn. 31, 1002–1006 (2011).
Keymolen, K., Staessen, C., Verpoest, W., Liebaers, I. & Bonduelle, M. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur. J. Hum. Genet. 20, 376–380 (2012).
Scriven, P. N., Flinter, F. A., Khalaf, Y., Lashwood, A. & Mackie Ogilvie, C. Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study. Eur. J. Hum. Genet. 21, 1035–1041 (2013).
Ko, D. S., Cho, J. W., Lee, H. S., Kim, J. Y., Kang, I. S., Yang, K. M. et al. Preimplantation genetic diagnosis outcomes and meiotic segregation analysis of robertsonian translocation carriers. Fertil. Steril. 99, 1369–1376 (2013).
Chen, C. K., Wu, D., Yu, H. T., Lin, C. Y., Wang, M. L., Yeh, H. Y. et al. Preimplantation genetic diagnosis by fluorescence in situ hybridization of reciprocal and Robertsonian translocations. Taiwan J. Obstet. Gynecol. 53, 48–52 (2014).
De Rycke, M., Belva, F., Goossens, V., Moutou, C., SenGupta, S. B., Traeger-Synodinos, J. et al. ESHRE PGD Consortium data collection XIII: cycles from January to December 2010 with pregnancy follow-up to October 2011. Hum. Reprod. 30, 1763–1789 (2015).
Dechanet, C., Castelli, C., Reyftmann, L., Hamamah, S., Hedon, B., Dechaud, H. et al. Do female translocations influence the ovarian response pattern to controlled ovarian stimulation in preimplantation genetic diagnosis? Hum. Reprod. 26, 1232–1240 (2011).
Mackie Ogilvie, C. & Scriven, P. N. Meiotic outcomes in reciprocal translocation carriers ascertained in 3-day human embryos. Eur. J. Hum. Genet. 10, 801–806 (2002).
Ye, Y., Qian, Y., Xu, C. & Jin, F. Meiotic segregation analysis of embryos from reciprocal translocation carriers in PGD cycles. Reprod. Biomed. Online 24, 83–90 (2012).
Hirshfeld-Cytron, J., Sugiura-Ogasawara, M. & Stephenson, M. D. Management of recurrent pregnancy loss associated with a parental carrier of a reciprocal translocation: a systematic review. Semin. Reprod. Med. 29, 470–481 (2011).
Ikuma, S., Sato, T., Sugiura-Ogasawara, M., Nagayoshi, M., Tanaka, A., Takeda, S. et al. Preimplantation genetic diagnosis and natural conception: a comparison of live birth rates in patients with recurrent pregnancy loss associated with translocation. PLoS ONE 10, e0129958 (2015).
Teramoto, S. & Kato, O. Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod. Biomed. Online 15, 134–148 (2007).
Zhang, J., Chang, L., Sone, Y. & Silber, S. Minimal ovarian stimulation (mini-IVF) for IVF utilizing vitrification and cryopreserved embryo transfer. Reprod. Biomed. Online 21, 485–495 (2010).
Kato, K., Takehara, Y., Segawa, T., Kawachiya, S., Okuno, T., Kobayashi, T. et al. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-centre, Japanese cohort. Reprod. Biol. Endocrinol. 10, 35 (2012).
Bodri, D., Kawachiya, S., De Brucker, M., Tournaye, H., Kondo, M., Kato, R. et al. Cumulative success rates following mild IVF in unselected infertile patients: a 3-year, single-centre cohort study. Reprod. Biomed. Online 28, 572–581 (2014).
Verlinsky, Y. An Atlas of Preimplantation Genetic Diagnosis 19–26 (Parthenon Publishing, New York, NY, USA, 2000).
Gianaroli, L., Magli, M. C., Ferraretti, A. P., Tabanelli, C., Trengia, V., Farfalli, V. et al. The beneficial effects of preimplantation genetic diagnosis for aneuploidy support extensive clinical application. Reprod. Biomed. Online 10, 633–640 (2005).
Velilla, E., Escudero, T. & Munne, S. Blastomere fixation techniques and risk of misdiagnosis for preimplantation genetic diagnosis of aneuploidy. Reprod. Biomed. Online 4, 210–217 (2002).
Aoyama, N., Kuwayama, M., Teramoto, S., Takehara, Y., Kawachiya, S. & Kato, O. A simple efficient method of blastomere fixation for preimplantation genetic diagnosis of aneuploidy with interphase FISH. Fertil. Steril. 86, S482–S483 (2006).
Yoshizawa, M., Konno, H., Zhu, S., Kageyama, S., Fukui, E., Muramatsu, S. et al. Chromosomal diagnosis in each individual blastomere of 5- to 10-cell bovine embryos derived from in vitro fertilization. Theriogenology 51, 1239–1250 (1999).
Kuwayama, M., Vajta, G., Kato, O. & Leibo, S. P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 11, 300–308 (2005).
Kato, K., Ueno, S., Yabuuchi, A., Uchiyama, K., Okuno, T., Kobayashi, T. et al. Women's age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod. Biomed. Online 29, 411–416 (2014).
Franssen, M. T., Korevaar, J. C., van der Veen, F., Leschot, N. J., Bossuyt, P. M. & Goddijn, M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ 332, 759–763 (2006).
Sugiura-Ogasawara, M., Ozaki, Y., Sato, T., Suzumori, N. & Suzumori, K. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocations. Fertil. Steril. 81, 367–373 (2004).
Lejeune, J. Autosomal disorders. Pediatrics 32, 326–337 (1963).
Anton, E., Vidal, F. & Blanco, J. Reciprocal translocations: tracing their meiotic behavior. Genet. Med. 10, 730–738 (2008).
Shi, Q. & Martin, R. H. Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction 121, 655–666 (2001).
Benet, J., Oliver-Bonet, M., Cifuentes, P., Templado, C. & Navarro, J. Segregation of chromosomes in sperm of reciprocal translocation carriers: a review. Cytogenet. Genome Res. 111, 281–290 (2005).
Douet-Guilbert, N., Bris, M. J., Amice, V., Marchetti, C., Delobel, B., Amice, J. et al. Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int. J. Androl. 28, 372–379 (2005).
Pujol, A., Benet, J., Staessen, C., Van Assche, E., Campillo, M., Egozcue, J. et al. The importance of aneuploidy screening in reciprocal translocation carriers. Reproduction 131, 1025–1035 (2006).
Godo, A., Blanco, J., Vidal, F., Sandalinas, M., Garcia-Guixé, E. & Anton, E. Altered segregation pattern and numerical chromosome abnormalities interrelate in spermatozoa from Robertsonian translocation carriers. Reprod. Biomed. Online 31, 79–88 (2015).
Dakov, T., Dimitrova, V. & Todorov, T. Pregnancy outcome in women over the age of 35. Akush Ginekol (Sofiia) 53, 9–14 (2014).
Prine, L. W. & MacNaughton, H. Office management of early pregnancy loss. Am. Fam. Physician 84, 75–82 (2011).
Wang, X., Chen, C., Wang, L., Chen, D., Guang, W. & French, J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil. Steril. 79, 577–584 (2003).
Everett, C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ 315, 32–34 (1997).
Nagaishi, M., Yamamoto, T., Iinuma, K., Shimomura, K., Berend, S. A. & Knops, J. Chromosome abnormalities identified in 347 spontaneous abortions collected in Japan. J. Obstet. Gynaecol. Res. 30, 237–241 (2004).
Jenderny, J. Chromosome aberrations in a large series of spontaneous miscarriages in the German population and review of the literature. Mol. Cytogenet. 7, 38 (2014).
Philipp, T., Philipp, K., Reiner, A., Beer, F. & Kalousek, D. K. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum. Reprod. 18, 1724–1732 (2003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kato, K., Aoyama, N., Kawasaki, N. et al. Reproductive outcomes following preimplantation genetic diagnosis using fluorescence in situ hybridization for 52 translocation carrier couples with a history of recurrent pregnancy loss. J Hum Genet 61, 687–692 (2016). https://doi.org/10.1038/jhg.2016.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.39
This article is cited by
-
Preimplantation genetic testing using comprehensive genomic copy number analysis is beneficial for balanced translocation carriers
Journal of Human Genetics (2024)
-
Nanopore sequencing for detecting reciprocal translocation carrier status in preimplantation genetic testing
BMC Genomics (2023)
-
De novo balanced reciprocal translocation mosaic t(1;3)(q42;q25) detected by prenatal genetic diagnosis: a fetus conceived using preimplantation genetic testing due to a t(12;14)(q22;q13) balanced paternal reciprocal translocation
Molecular Cytogenetics (2021)
-
Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR)
Journal of Assisted Reproduction and Genetics (2019)
-
Pregnancy outcomes of reciprocal translocation carriers with two or more unfavorable pregnancy histories: before and after preimplantation genetic testing
Journal of Assisted Reproduction and Genetics (2019)