Abstract
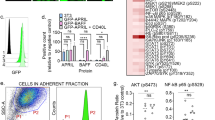
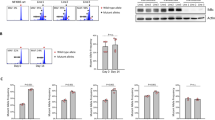
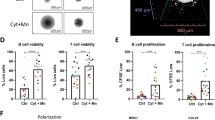
Chronic lymphocytic leukemia (CLL) cells depend on microenvironmental factors for proliferation and survival. In particular, the B-cell receptor (BCR) and nuclear factor- κB (NF-κB) pathways are activated in the lymph node (LN) microenvironment. Thus, model systems mimicking tumor–host interactions are important tools to study CLL biology and pathogenesis. We investigated whether the recently established NOD/scid/γcnull (NSG) mouse xenograft model can recapitulate the effects of the human microenvironment. We assessed, therefore, tumor characteristics previously defined in LN-resident CLL cells, including proliferation, and activation of the BCR and NF-κB pathways. We found that the murine spleen (SP) microenvironment supported CLL cell proliferation and activation to a similar degree than the human LN, including induction of BCR and NF-κB signaling in the xenografted cells. Next, we used this model to study ibrutinib, a Bruton’s tyrosine kinase inhibitor in clinical development. Ibrutinib inhibited BCR and NF-κB signaling induced by the microenvironment, decreased proliferation, induced apoptosis and reduced the tumor burden in vivo. Thus, our data demonstrate that the SP of xenografted NSG mice can, in part, recapitulate the role of the human LN for CLL cells. In addition, we show that ibrutinib effectively disrupts tumor–host interactions essential for CLL cell proliferation and survival in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ghia P, Chiorazzi N, Stamatopoulos K . Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med 2008; 264: 549–562.
Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D et al. in vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest 2005; 115: 755–764.
Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011; 117: 563–574.
Chiorazzi N . Cell proliferation and death: forgotten features of chronic lymphocytic leukemia B cells. Best Pract Res Clin Haematol 2007; 20: 399–413.
Deaglio S, Malavasi F . Chronic lymphocytic leukemia microenvironment: shifting the balance from apoptosis to proliferation. Haematologica 2009; 94: 752–756.
Gine E, Martinez A, Villamor N, Lopez-Guillermo A, Camos M, Martinez D et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia ("accelerated" chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica 2010; 95: 1526–1533.
Burger JA . Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 2011; 2011: 96–103.
Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ . Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000; 96: 2655–2663.
Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood 2001; 98: 3050–3057.
Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG . The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood 2008; 111: 846–855.
Muzio M, Scielzo C, Bertilaccio MT, Frenquelli M, Ghia P, Caligaris-Cappio F . Expression and function of toll like receptors in chronic lymphocytic leukaemia cells. Br J Haematol 2009; 144: 507–516.
Luqman M, Klabunde S, Lin K, Georgakis GV, Cherukuri A, Holash J et al. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood 2008; 112: 711–720.
Endo T, Nishio M, Enzler T, Cottam HB, Fukuda T, James DF et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood 2007; 109: 703–710.
Buchner M, Baer C, Prinz G, Dierks C, Burger M, Zenz T et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood 2010; 115: 4497–4506.
Wiestner A . Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood 2012; 120: 4684–4691.
Woyach JA, Johnson AJ, Byrd JC . The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 2012; 120: 1175–1184.
Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest 1998; 102: 1515–1525.
Tobin G, Thunberg U, Karlsson K, Murray F, Laurell A, Willander K et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood 2004; 104: 2879–2885.
Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood 2012; 119: 4467–4475.
Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med 2004; 200: 519–525.
Chu CC, Catera R, Hatzi K, Yan XJ, Zhang L, Wang XB et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood 2008; 112: 5122–5129.
Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK . Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood 2007; 109: 4424–4431.
Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood 2008; 112: 188–195.
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31: 88–94.
Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010; 115: 2578–2585.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Sharman JP et al. The Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 (P) in treatment-naive (TN) chronic lymphocytic leukemia (CLL) patients (pts): Interim results of a phase Ib/II study. J Clin Oncol 2012; 30 (suppl): )abstr 6507.
Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM Jr., Wagner-Johnston ND et al. CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110{delta}, demonstrates clinical activity and pharmacodynamic effects in patients with relapsed or refractory chronic lymphocytic leukemia. ASH Annual Meeting Abstracts 2010; 116: 55.
O’Brien S, Burger JA, Blum KA, Furman RR, Coutre SE, Sharman J et al. The Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 induces durable responses in relapsed or refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): follow-up of a phase Ib/II study. ASH Ann Meet Abs 2011; 118: 983.
Wiestner A . Targeting B-cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol 2013; 31: 128–130.
Cheson BD, Byrd JC, Rai KR, Kay NE, O’Brien SM, Flinn IW et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol 2012; 30: 2820–2822.
Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011; 117: 6287–6296.
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA 2010; 107: 13075–13080.
Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119: 1182–1189.
de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012; 119: 2590–2594.
Herman SE, Barr PM, McAuley EM, Liu D, Wiestner A, Friedberg JW . Fostamatinib inhibits B-cell receptor signaling, cellular activation and tumor proliferation in patients with relapsed and refractory chronic lymphocytic leukemia. Leukemia 2013; e-pub ahead of print 6 February 2013; doi:10.1038/leu.2013.37.
Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA 2002; 99: 6955–6960.
Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 2010; 17: 28–40.
Bertilaccio MT, Scielzo C, Simonetti G, Ponzoni M, Apollonio B, Fazi C et al. A novel Rag2-/-gammac-/—xenograft model of human CLL. Blood 2010; 115: 1605–1609.
Bagnara D, Kaufman MS, Calissano C, Marsilio S, Patten PE, Simone R et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood 2011; 117: 5463–5472.
Durig J, Ebeling P, Grabellus F, Sorg UR, Mollmann M, Schutt P et al. A novel nonobese diabetic/severe combined immunodeficient xenograft model for chronic lymphocytic leukemia reflects important clinical characteristics of the disease. Cancer Res 2007; 67: 8653–8661.
Aydin S, Grabellus F, Eisele L, Mollmann M, Hanoun M, Ebeling P et al. Investigating the role of CD38 and functionally related molecular risk factors in the CLL NOD/SCID xenograft model. Eur J Haematol 2011; 87: 10–19.
Biagi E, Dotti G, Yvon E, Lee E, Pule M, Vigouroux S et al. Molecular transfer of CD40 and OX40 ligands to leukemic human B cells induces expansion of autologous tumor-reactive cytotoxic T lymphocytes. Blood 2005; 105: 2436–2442.
Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther 2011; 13: R115.
Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003; 3: 185–197.
Suljagic M, Longo PG, Bennardo S, Perlas E, Leone G, Laurenti L et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eμ- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood 2010; 116: 4894–4905.
Rosen A, Murray F, Evaldsson C, Rosenquist R . Antigens in chronic lymphocytic leukemia—implications for cell origin and leukemogenesis. Semin Cancer Biol 2010; 20: 400–409.
Duhren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012; 489: 309–312.
Efremov D, Wiestner A, Laurenti L . Novel agents and emerging strategies for targeting the B-cell receptor pathway in CLL. Mediterr J Hematol Infect Dis 2012; 4: e2012067.
Acknowledgements
We thank Dr Nalini Raghavachari for her assistance setting up the Taqman microfluidics cards, Dr Zu Xi Yu and the NHLBI pathology core for help with slide preparation, and Christina Corsino and the NIAID building 50 animal technicians for their assistance with animal experiments. This research was funded through the Intramural Research Program of the National, Heart, Lung and Blood Institute.
Author contributions
SEMH, XS, EMM and PP-G, planned the research, performed experiments and analyzed data; MMH, KK, CMC, JFT, JC and GA were involved in planning components of the research, contributed reagents and supported experiments; SP and MR provided expert pathology services; DL conducted statistical analyses; JJB was involved in planning components of the research, provided essential reagents, and reviewed the draft manuscript; AW planned and supervised the research, and analyzed data. SEMH, XS, PP-G and AW wrote the paper. All authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JJB is an employee of Pharmacyclics Inc. and has financial interest in ibrutinib development. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Herman, S., Sun, X., McAuley, E. et al. Modeling tumor–host interactions of chronic lymphocytic leukemia in xenografted mice to study tumor biology and evaluate targeted therapy. Leukemia 27, 2311–2321 (2013). https://doi.org/10.1038/leu.2013.131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.131
Keywords
This article is cited by
-
A Comprehensive Review of Cancer Drug–Induced Cardiotoxicity in Blood Cancer Patients: Current Perspectives and Therapeutic Strategies
Current Treatment Options in Oncology (2024)
-
Overexpression of wild type RRAS2, without oncogenic mutations, drives chronic lymphocytic leukemia
Molecular Cancer (2022)
-
Machine learning can identify newly diagnosed patients with CLL at high risk of infection
Nature Communications (2020)
-
Targeting B cell receptor signalling in cancer: preclinical and clinical advances
Nature Reviews Cancer (2018)
-
Activity of the novel BCR kinase inhibitor IQS019 in preclinical models of B-cell non-Hodgkin lymphoma
Journal of Hematology & Oncology (2017)