Abstract
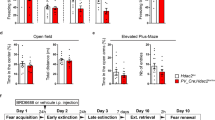
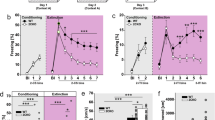
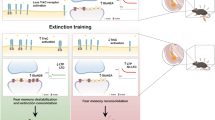
Critical periods are temporary windows of heightened neural plasticity early in development. For example, fear memories in juvenile rodents are subject to erasure following extinction training, while after closure of this critical period, extinction training only temporarily and weakly suppresses fear memories. Persistence of fear memories is important for survival, but the inability to effectively adapt to the trauma is a characteristic of post-traumatic stress disorder (PTSD). We examined whether Nogo Receptor 1 (NgR1) regulates the plasticity associated with fear extinction. The loss of NgR1 function in adulthood eliminates spontaneous fear recovery and fear renewal, with a restoration of fear reacquisition rate equal to that of naive mice; thus, mimicking the phenotype observed in juvenile rodents. Regional gene disruption demonstrates that NgR1 expression is required in both the basolateral amygdala (BLA) and infralimbic (IL) cortex to prevent fear erasure. NgR1 expression by parvalbumin expressing interneurons is essential for limiting extinction-dependent plasticity. NgR1 gene deletion enhances anatomical changes of inhibitory synapse markers after extinction training. Thus, NgR1 robustly inhibits elimination of fear expression in the adult brain and could serve as a therapeutic target for anxiety disorders, such as PTSD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kim JH, Richardson R . A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci 2007; 121: 131–139.
Kim JH, Richardson R . A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem 2007; 88: 48–57.
Gogolla N, Caroni P, Luthi A, Herry C . Perineuronal nets protect fear memories from erasure. Science 2009; 325: 1258–1261.
Pavlov IP . Conditioned Reflexes Oxford UP: London, 1927.
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A . Neuronal circuits of fear extinction. Eur J Neurosci 2010; 31: 599–612.
Bouton ME . Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 2002; 52: 976–986.
Quartermain D, McEwen BS . Temporal characteristics of amnesia induced by protein synthesis inhibitor: determination by shock level. Nature 1970; 228: 677–678.
Quartermain D, McEwen BS, Azmitia EC Jr . Amnesia produced by electroconvulsive shock or cycloheximide: conditions for recovery. Science 1970; 169: 683–686.
Fournier AE, GrandPre T, Strittmatter SM . Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001; 409: 341–346.
Liu BP, Fournier A, GrandPre T, Strittmatter SM . Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 2002; 297: 1190–1193.
Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 2012; 15: 703–712.
Wang X, Chun S, Treloar H, Vartanian T, Greer C, Strittmatter S . Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci 2002; 22: 5505–5515.
Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ . Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci 2010; 30: 12432–12445.
McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM . Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 2005; 309: 2222–2226.
Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM . Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron 2013; 77: 859–866.
Zemmar A, Weinmann O, Kellner Y, Yu X, Vicente R, Gullo M et al. Neutralization of Nogo-A enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J Neurosci 2014; 34: 8685–8698.
Karlen A, Karlsson TE, Mattsson A, Lundstromer K, Codeluppi S, Pham TM et al. Nogo receptor 1 regulates formation of lasting memories. Proc Natl Acad Sci USA 2009; 106: 20476–20481.
Budel S, Padukkavidana T, Liu BP, Feng Z, Hu FH, Johnson S et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci 2008; 28: 13161–13172.
Kim JE, Li SX, GrandPre T, Qiu D, Strittmatter SM . Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 2003; 38: 187–199.
Kim JE, Liu BP, Park JH, Strittmatter SM . Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 2004; 44: 439–451.
Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 2005; 3: e159.
Wang X, Duffy P, McGee AW, Hasan O, Gould G, Tu N et al. Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann Neurol 2011; 70: 805–821.
Wang X, Yigitkanli K, Kim CY, Sekine-Komo T, Wirak D, Frieden E et al. Human NgR-Fc decoy protein via lumbar intrathecal bolus administration enhances recovery from rat spinal cord contusion. J Neurotrauma 2014; 31: 1955–1966.
Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci 2006; 26: 1386–1395.
Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y . Long-lasting incubation of conditioned fear in rats. Biol Psychiatry 2009; 65: 881–886.
Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE . Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci 2007; 10: 414–416.
Diaz-Mataix L, Debiec J, LeDoux JE, Doyere V . Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci 2011; 31: 9538–9543.
Lai CS, Franke TF, Gan WB . Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 2012; 483: 87–91.
Liu BP, Fournier A, GrandPre T, Strittmatter SM . Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor.[comment]. Science 2002; 297: 1190–1193.
Fournier AE, Gould GC, Liu BP, Strittmatter SM . Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci 2002; 22: 8876–8883.
Li SX, Liu BP, Budel S, Li MW, Ji BX, Walus L et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci 2004; 24: 10511–10520.
Rodrigues SM, Schafe GE, LeDoux JE . Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 2004; 44: 75–91.
Duvarci S, Pare D . Amygdala microcircuits controlling learned fear. Neuron 2014; 82: 966–980.
Barad M, Gean PW, Lutz B . The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry 2006; 60: 322–328.
Quirk GJ, Russo GK, Barron JL, Lebron K . The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 2000; 20: 6225–6231.
Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ . Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem 2006; 13: 728–733.
Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ . Induction of fear extinction with hippocampal-infralimbic BDNF. Science 2010; 328: 1288–1290.
Trouche S, Sasaki JM, Tu T, Reijmers LG . Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 2013; 80: 1054–1065.
Donato F, Rompani SB, Caroni P . Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 2013; 504: 272–276.
Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 2014; 505: 92–96.
Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 2014; 509: 453–458.
Josephson A, Trifunovski A, Scheele C, Widenfalk J, Wahlestedt C, Brene S et al. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res 2003; 311: 333–342.
Stephany CE, Chan LL, Parivash SN, Dorton HM, Piechowicz M, Qiu S et al. Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J Neurosci 2014; 34: 11631–11640.
Gourley SL, Kedves AT, Olausson P, Taylor JR . A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 2009; 34: 707–716.
Milad MR, Quirk GJ . Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012; 63: 129–151.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE . Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897–905.
Chhatwal JP, Myers KM, Ressler KJ, Davis M . Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci 2005; 25: 502–506.
Heldt SA, Ressler KJ . Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci 2007; 26: 3631–3644.
Hensch TK . Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6: 877–888.
Acknowledgements
SMS is a member of the Kavli Institute for Neuroscience at Yale University. We acknowledge research support from the NIH and the Falk Medical Research Trust to SMS. We thank Adam Kaufman, Stefano Sodi and Yiguang Fu for technical assistance. SMB is supported by the National Science Foundation Graduate Research Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SMS is a cofounder of Axerion Therapeutics, seeking to develop PrP- and NgR-based therapeutics.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Bhagat, S., Butler, S., Taylor, J. et al. Erasure of fear memories is prevented by Nogo Receptor 1 in adulthood. Mol Psychiatry 21, 1281–1289 (2016). https://doi.org/10.1038/mp.2015.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.179
This article is cited by
-
Acan downregulation in parvalbumin GABAergic cells reduces spontaneous recovery of fear memories
Molecular Psychiatry (2023)
-
Neuroregeneration and plasticity: a review of the physiological mechanisms for achieving functional recovery postinjury
Military Medical Research (2020)
-
Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex
Brain Structure and Function (2020)
-
Activity-dependent modulation of hippocampal synaptic plasticity via PirB and endocannabinoids
Molecular Psychiatry (2019)
-
Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor
Molecular Psychiatry (2017)