Abstract
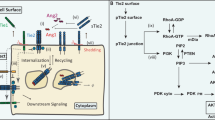
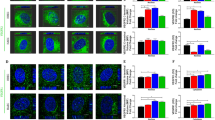
The receptor tyrosine kinase Tie2, and its activating ligand Angiopoietin-1 (Ang1), are required for vascular remodelling and vessel integrity, whereas Ang2 may counteract these functions. However, it is not known how Tie2 transduces these different signals. Here, we show that Ang1 induces unique Tie2 complexes in mobile and confluent endothelial cells. Matrix-bound Ang1 induced cell adhesion, motility and Tie2 activation in cell–matrix contacts that became translocated to the trailing edge in migrating endothelial cells. In contrast, in contacting cells Ang1 induced Tie2 translocation to cell–cell contacts and the formation of homotypic Tie2–Tie2 trans-associated complexes that included the vascular endothelial phosphotyrosine phosphatase, leading to inhibition of paracellular permeability. Distinct signalling proteins were preferentially activated by Tie2 in the cell–matrix and cell–cell contacts, where Ang2 inhibited Ang1-induced Tie2 activation. This novel type of cellular microenvironment-dependent receptor tyrosine kinase activation may explain some of the effects of angiopoietins in angiogenesis and vessel stabilization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dumont, D. J. et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes. Dev. 8, 1897–1909 (1994).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996).
Sato, T. N. et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 367, 70–74 (1995).
Yancopoulos, G. D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Peters, K. G. et al. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog. Horm. Res. 59, 51–71 (2004).
Brindle, N. P., Saharinen, P. & Alitalo, K. Signaling and functions of angiopoietin-1 in vascular protection. Circ. Res. 98, 1014–1023 (2006).
Eklund, L. & Olsen, B. R. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 312, 630–641 (2006).
Pfaff, D., Fiedler, U. & Augustin, H. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J. Leukocyte Biol. 80, 719–726 (2006).
Wong, A. L. et al. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ. Res. 81, 567–574 (1997).
Thurston, G. et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286, 2511–2514 (1999).
Davis, S. et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87, 1161–1169 (1996).
Koblizek, T. I., Weiss, C., Yancopoulos, G. D., Deutsch, U. & Risau, W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 8, 529–532 (1998).
Papapetropoulos, A. et al. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Invest. 79, 213–223 (1999).
Saharinen, P. et al. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J. Cell Biol. 169, 239–243 (2005).
Maisonpierre, P.C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Teichert-Kuliszewska, K. et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc. Res. 49, 659–670 (2001).
Daly, C. et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc. Natl Acad. Sci. USA 103, 15491–15496 (2006).
Bogdanovic, E., Nguyen, V. P. & Dumont, D. J. Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J. Cell Sci. 119, 3551–3560 (2006).
Cho, C. H. et al. COMP–Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc. Natl Acad. Sci. USA 101, 5547–5552 (2004).
Cho, C. H. et al. Designed angiopoietin-1 variant, COMP–Ang1, protects against radiation-induced endothelial cell apoptosis. Proc. Natl Acad. Sci. USA 101, 5553–5558 (2004).
Marron, M., Hughes, D. P., Edge, M. D., Forder, C. L. & Brindle, N. Evidence for heterotypic interaction between the receptor tyrosine kinases TIE-1 and TIE-2. J. Biol. Chem. 275, 39741–39746 (2000).
Yuan, H. T.. et al. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 21, 3171–3178 (2007).
Lampugnani, M. G. et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE–cadherin). J. Cell Biol. 129, 203–217 (1995).
Jones, N. et al. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol. Cell. Biol. 23, 2658–2668 (2003).
Beardsley, A. et al. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J. Biol. Chem. 280, 3541–3547 (2005).
Grande-Garcia, A. et al. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 177, 683–694 (2007).
Xu, Y. & Yu, Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J. Biol. Chem. 276, 34990–34998 (2001).
Fachinger, G., Deutsch, U. & Risau, W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene 18, 5948–5953 (1999).
Gamble, J. R. et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 87, 603–607 (2000).
Nawroth, R. et al. VE–PTP and VE–cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 21, 4885–4895 (2002).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Master, Z. et al. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J. 20, 5919–5928 (2001).
Kim, I. et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3´-Kinase/Akt signal transduction pathway. Circ. Res. 86, 24–29 (2000).
Hanahan, D. Signaling vascular morphogenesis and maintenance. Science 277, 48–50 (1997).
Stratmann, A., Risau, W. & Plate, K. H. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am. J. Pathol. 153, 1459–1466 (1998).
Holash, J. et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998 (1999).
Edgell, C. J., McDonald, C. C. & Graham, J. B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl Acad. Sci. USA 80, 3734–3737 (1983).
Lieber, M., Smith, B., Szakal, A., Nelson-Rees, W. & Todaro, G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 17, 62–70 (1976).
Ory, D. S., Neugeboren, B. A. & Mulligan, R. C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl Acad. Sci. USA 93, 11400–11406 (1996).
Salven, P. et al. Endothelial Tie growth factor receptor provides antigenic marker for assessment of breast cancer angiogenesis. Br. J. Cancer 74, 69–72 (1996).
Andriopoulou, P., Navarro, P., Zanetti, A., Lampugnani, M. G. & Dejana, E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc. Biol. 19, 2286–2297 (1999).
Kim, K. T. et al. Oligomerization and multimerization is critical for angiopoietin-1 to bind and phosphorylate tie2. J. Biol. Chem. 280, 20126–20131 (2005).
Acknowledgements
We thank: D. Dumont for the Dok-R construct; M. Jeltsch for VEGF-C; J. Partanen and H. Augustin for constructive criticism on the manuscript; N. Ihalainen, T. LaakkonenV. Pihlajaniemi, K. Pulkki and J. Träskelin for excellent technical assistance; S. Eskelinen, A. Manninen and the Molecular Imaging Unit of Biomedicum Helsinki for advice. This work was supported by grants from the the Academy of Finland (213327 (P. S, 116138 (L.E.), 202852, 204312 (K. A.)), the Louis Jeantet Foundation, the Human Frontier Science Program, the Finnish Cancer Organizations, the Novo Nordisk Foundation, National Institute of Health grants HL075183-02 (K. A.), AR36820 and AR48564 (B. R. O.) and by the KOSEF through the NRL (2004-02376, G. Y. K.) funded by the MOST.
Author information
Authors and Affiliations
Contributions
P. S., L. E. and K. A. designed most of the experiments; P. S. and L. E. participated in the experimental work, performed most of the data collection, data analysis. writing and processing of figures; J. M. and R. W. performed a significant amount of the experimental work and some planning of experiments; A. A. participated in the S2 cell experiments; M. W., A. N. and D. W. performed VE-PTP staining of mouse endothelioma cells and produced anti-VE-PTP anti-serum; U. D. provided Tie2−/− and Tie2+/+ endothelioma cells; G. Y. K. provided COMP–Ang1; U. D. and G. Y. K. provided advice. Most of the work was carried out in the laboratories of L. E. and K. A., and some experiments in the laboratory of B. R. O.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5, S6, S7, S8 and Supplemntary Methods (PDF 7136 kb)
Supplementary Information
Supplementary Movie 1 (MOV 1156 kb)
Supplementary Information
Supplementary Movie 2 (MOV 2322 kb)
Supplementary Information
Supplementary Movie 3 (MOV 3359 kb)
Supplementary Information
Supplementary Movie 4 (MOV 640 kb)
Supplementary Information
Supplementary Movie 5 (MOV 279 kb)
Supplementary Information
Supplementary Movie 6 (MOV 2496 kb)
Supplementary Information
Supplementary Movie 7 (MOV 1151 kb)
Rights and permissions
About this article
Cite this article
Saharinen, P., Eklund, L., Miettinen, J. et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat Cell Biol 10, 527–537 (2008). https://doi.org/10.1038/ncb1715
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1715
This article is cited by
-
Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury
Nature Reviews Nephrology (2022)
-
In vitro inhibition of cancer angiogenesis and migration by a nanobody that targets the orphan receptor Tie1
Cellular and Molecular Life Sciences (2022)
-
Discontinuation and loss to follow-up rates in clinical trials of intravitreal anti-vascular endothelial growth factor injections
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
The Role of Angiopoietins in Neovascular Diabetes-Related Retinal Diseases
Diabetes Therapy (2022)
-
DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling
Stem Cell Research & Therapy (2021)