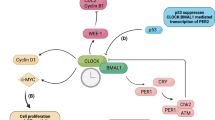
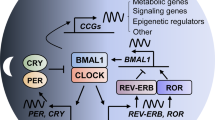
Abstract
Virtually all cells in the body have an intracellular clockwork based on a negative feedback mechanism. The circadian timekeeping system in mammals is a hierarchical multi-oscillator network, with the suprachiasmatic nuclei (SCN) acting as the central pacemaker. The SCN synchronizes to daily light-dark cycles and coordinates rhythmic physiology and behavior. Synchronization in the SCN and at the organismal level is a key feature of the circadian clock system. In particular, intercellular coupling in the SCN synchronizes neuron oscillators and confers robustness against perturbations. Recent advances in our knowledge of and ability to manipulate circadian rhythms make available cell-based clock models, which lack strong coupling and are ideal for target discovery and chemical biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Toh, K.L. et al. An hPer2 phosphorylation site mutation in familiar advanced sleep phase syndrome. Science 291, 1040–1043 (2001).
Xu, Y. et al. Functional consequences of a CKI delta mutation causing familial advanced sleep phase syndrome. Nature 434, 640–644 (2005).
Ueda, H.R. et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 37, 187–192 (2005).
Young, M.W. & Kay, S.A. Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2, 702–715 (2001).
Reppert, S.M. & Weaver, D.R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
Gallego, M. & Virshup, D.M. Post-translational modifications relgulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 8, 139–148 (2007).
Yoo, S.H. et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 101, 5339–5346 (2004).
Welsh, D.K., Yoo, S.H., Liu, A.C., Takahashi, J.S. & Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 14, 2289–2295 (2004).
Liu, A.C. et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129, 605–616 (2007).
Nagoshi, E. et al. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119, 693–705 (2004).
van den Pol, A.N. & Dudek, F.E. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience 56, 793–811 (1993).
Low-Zeddies, S.S. & Takahashi, J.S. Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell 105, 25–42 (2001).
Welsh, D.K., Logothetis, D.E., Meister, M. & Reppert, S.M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706 (1995).
Aton, S.J. & Herzog, E.D. Come together, right...now: synchronization of rhythms in a mammalian circadian clock. Neuron 48, 531–534 (2005).
Nakamura, W., Honma, S., Shirakawa, T. & Honma, K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat. Neurosci. 5, 399–400 (2002).
Herzog, E.D., Aton, S.J., Numano, R., Sakaki, Y. & Tei, H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J. Biol. Rhythms 19, 35–46 (2004).
Horikawa, K., Ishimatsu, K., Yoshimoto, E., Kondo, S. & Takeda, H. Noise-resistant and synchronized oscillation of the segmentation clock. Nature 441, 719–723 (2006).
Masamizu, Y. et al. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc. Natl. Acad. Sci. USA 103, 1313–1318 (2006).
Foster, R.G. et al. Circadian photoreception in the retinally degenerate mouse (rd/rd). J. Comp. Physiol. [A] 169, 39–50 (1991).
Panda, S. et al. Illumination of the melanopsin signaling pathway. Science 307, 600–604 (2005).
Melyan, Z., Tarttelin, E.E., Bellingham, J., Lucas, R.J. & Hankins, M.W. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745 (2005).
Qiu, X. et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature 433, 745–749 (2005).
Dacey, D.M. et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (2005).
Hattar, S., Liao, H.W., Takao, M., Berson, D.M. & Yau, K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Berson, D.M., Dunn, F.A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Freedman, M.S. et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504 (1999).
Panda, S. et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216 (2002).
Ruby, N.F. et al. Role of melanopsin in circadian responses to light. Science 298, 2211–2213 (2002).
Lucas, R.J. et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247 (2003).
Hattar, S. et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003).
Panda, S. et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 (2003).
Meijer, J.H. & Schwartz, W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythms 18, 235–249 (2003).
Antle, M.C. & Silver, R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 28, 145–151 (2005).
Morris, M.E., Viswanathan, N., Kuhlman, S., Davis, F.C. & Weitz, C.J. A screen for genes induced in the suprachiasmatic nucleus by light. Science 279, 1544–1547 (1998).
Ginty, D.D. et al. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260, 238–241 (1993).
Travnickova-Bendova, Z., Cermakian, N., Reppert, S.M. & Sassone-Corsi, P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc. Natl. Acad. Sci. USA 99, 7728–7733 (2002).
Tischkau, S.A., Mitchell, J.W., Tyan, S.H., Buchanan, G.F. & Gillette, M.U. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J. Biol. Chem. 278, 718–723 (2003).
Gillette, M.U. & Mitchell, J.W. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 309, 99–107 (2002).
Tischkau, S.A. et al. Protein kinase G type II is required for night-to-day progression of the mammalian circadian clock. Neuron 43, 539–549 (2004).
Yamaguchi, S. et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302, 1408–1412 (2003).
Albus, H. et al. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr. Biol. 12, 1130–1133 (2002).
Liu, C., Weaver, D.R., Strogatz, S.H. & Reppert, S.M. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell 91, 855–860 (1997).
Herzog, E.D., Takahashi, J.S. & Block, G.D. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1, 708–713 (1998).
Liu, C. & Reppert, S.M. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25, 123–128 (2000).
Albus, H., Vansteensel, M.J., Michel, S., Block, G.D. & Meijer, J.H.A. GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr. Biol. 15, 886–893 (2005).
Harmar, A.J. et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109, 497–508 (2002).
Colwell, C.S. et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R939–R949 (2003).
Aton, S.J., Colwell, C.S., Harmar, A.J., Waschek, J. & Herzog, E.D. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 8, 476–483 (2005).
Maywood, E.S. et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol. 16, 599–605 (2006).
Cutler, D.J. et al. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur. J. Neurosci. 17, 197–204 (2003).
Aida, R. et al. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol. Pharmacol. 61, 26–34 (2002).
Piggins, H.D., Antle, M.C. & Rusak, B. Neuropeptides phase shift the mammalian circadian pacemaker. J. Neurosci. 15, 5612–5622 (1995).
McArthur, A.J. et al. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J. Neurosci. 20, 5496–5502 (2000).
Brown, T.M., Hughes, A.T. & Piggins, H.D. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J. Neurosci. 25, 11155–11164 (2005).
Long, M.A., Jutras, M.J., Connors, B.W. & Burwell, R.D. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat. Neurosci. 8, 61–66 (2005).
Nitabach, M.N., Blau, J. & Holmes, T.C. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109, 485–495 (2002).
Aton, S.J., Huettner, J.E., Straume, M. & Herzog, E.D. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc. Natl. Acad. Sci. USA 103, 19188–19193 (2006).
Kuhlman, S.J. & McMahon, D.G. Encoding the ins and outs of circadian pacemaking. J. Biol. Rhythms 21, 470–481 (2006).
Buijs, R.M. & Kalsbeek, A. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci. 2, 521–526 (2001).
Kalsbeek, A. et al. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 21, 458–469 (2006).
Schibler, U., Ripperger, J. & Brown, S.A. Peripheral circadian oscillators in mammals: time and food. J. Biol. Rhythms 18, 250–260 (2003).
Silver, R., LeSauter, J., Tresco, P.A. & Lehman, M.N. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813 (1996).
Pando, M.P., Morse, D., Cermakian, N. & Sassone-Corsi, P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell 110, 107–117 (2002).
Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002).
Abrahamson, E.E., Leak, R.K. & Moore, R.Y. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12, 435–440 (2001).
Jin, X. et al. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96, 57–68 (1999).
Tousson, E. & Meissl, H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J. Neurosci. 24, 2983–2988 (2004).
Kramer, A. et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294, 2511–2515 (2001).
Cheng, M.Y. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410 (2002).
Kraves, S. & Weitz, C.J. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat. Neurosci. 9, 212–219 (2006).
Prosser, H.M. et al. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc. Natl. Acad. Sci. USA 104, 648–653 (2007).
Li, J.D. et al. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J. Neurosci. 26, 11615–11623 (2006).
Balsalobre, A. et al. Resetting of circadian time peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 (2000).
Jusko, W.J. Pharmacokinetics and receptor-mediated pharmacodynamics of corticosteroids. Toxicology 102, 189–196 (1995).
Hastings, M.H., Reddy, A.B. & Maywood, E.S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4, 649–661 (2003).
Labrecque, G., Bureau, J.P. & Reinberg, A.E. Biological rhythms in the inflammatory response and in the effects of nonsteroidal antiinflammatory drugs. Pharmacol. Ther. 66, 285–300 (1995).
Mormont, M.C. & Levi, F. Cancer chronotherapy: principles, applications, and perspectives. Cancer 97, 155–169 (2003).
Levi, F. & Schibler, U. Circadian rhythms: mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 47, 593–628 (2007).
Pace-Schott, E.F. & Hobson, J.A. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 3, 591–605 (2002).
Dijk, D. & von Schantz, M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J. Biol. Rhythms 20, 279–290 (2005).
Morin, A.K., Jarvis, C.I. & Lynch, A.M. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacotherapy 27, 89–110 (2007).
Atack, J.R. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin. Investig. Drugs 14, 601–618 (2005).
Borjigin, J., Li, X.D. & Snyder, S.H. The pineal gland and melatonin: molecular and pharmacologic regulation. Annu. Rev. Pharmacol. Toxicol. 39, 53–65 (1999).
Kato, K. et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48, 301–310 (2005).
Carrillo-Vico, A., Guerrero, J.M., Lardone, P.J. & Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 27, 189–200 (2005).
McClung, C.A. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Ther. 114, 222–232 (2007).
Yin, L., Wang, J., Klein, P.S. & Lazar, M.A. Nuclear receptor Rev-erba is a critical lithium-sensitive component of the circadian clock. Science 311, 1002–1005 (2006).
Preitner, N. et al. The orphan nuclear receptor REV-ERB alpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Iitaka, C., Miyazaki, K., Akaike, T. & Ishida, N. A role for glycogen synthase kinase-3 beta in the mammalian circadian clock. J. Biol. Chem. 280, 29397–29402 (2005).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Stokkan, K.A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001).
Vitaterna, M.H. et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc. Natl. Acad. Sci. USA 103, 9327–9332 (2006).
Brisbare-Roch, C. et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat. Med. 13, 150–155 (2007).
Takahashi, J.S. Finding new clock components: past and future. J. Biol. Rhythms 19, 339–347 (2004).
Brown, S.A. et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 3, e338 (2005).
Missbach, M. et al. Thiazolidine diones, specific ligands of the nuclear receptor retinoid Z receptor/retinoid acid receptor-related orphan receptor alpha with potent antiarthritic activity. J. Biol. Chem. 271, 13515–13522 (1996).
Littman, D.R. et al. The role of the nuclear hormone receptor ROR gamma in the development of lymph nodes and Peyer's patches. Immunol. Rev. 195, 81–90 (2003).
Boukhtouche, F., Mariani, J. & Tedgui, A. The “CholesteROR” protective pathway in the vascular system. Arterioscler. Thromb. Vasc. Biol. 24, 637–643 (2004).
Eide, E.J. et al. Control of mammalian circadian rhythm by CKI epsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 25, 2795–2807 (2005).
Siepka, S.M. et al. The circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryprochrome and period gene expression. Cell 129, 1011–1023 (2007).
Busino, L. et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316, 900–904 (2007).
Godinho, S.I.H. et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316, 897–900 (2007).
Kornmann, B., Schaad, O., Bujard, H., Takahashi, J.S. & Schibler, U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 5, e34 (2007).
Brown, S.A., Zumbrunn, G., Fleury-Olela, F., Preitner, N. & Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 12, 1574–1583 (2002).
Acknowledgements
We thank D.K. Welsh for helpful discussion and critical reading of the manuscript. This work was supported in part by grants from the US National Institutes of Health (R01 GM074868 and R01 MH051573 to S.A.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Liu, A., Lewis, W. & Kay, S. Mammalian circadian signaling networks and therapeutic targets. Nat Chem Biol 3, 630–639 (2007). https://doi.org/10.1038/nchembio.2007.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2007.37
This article is cited by
-
Circadian rhythms in the blood–brain barrier: impact on neurological disorders and stress responses
Molecular Brain (2023)
-
Generation of CRISPR-Cas9-mediated knockin mutant models in mice and MEFs for studies of polymorphism in clock genes
Scientific Reports (2023)
-
Cloning, tissue distribution, mRNA expression and functional analysis of circadian clock gene per2 from the high-latitude Amur minnow (Phoxinus lagowskii)
Aquaculture International (2023)
-
Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: a friend or foe?
Acta Pharmacologica Sinica (2022)
-
Circadian rhythm as a therapeutic target
Nature Reviews Drug Discovery (2021)