Abstract
Methionine restriction extends the lifespan of various model organisms. Limiting S-adenosyl-methionine (SAM) synthesis, the first metabolic reaction of dietary methionine, extends longevity in Caenorhabditis elegans but accelerates pathology in mammals. Here, we show that, as an alternative to inhibiting SAM synthesis, enhancement of SAM catabolism by glycine N-methyltransferase (Gnmt) extends the lifespan in Drosophila. Gnmt strongly buffers systemic SAM levels by producing sarcosine in either high-methionine or low-sams conditions. During ageing, systemic SAM levels in flies are increased. Gnmt is transcriptionally induced in a dFoxO-dependent manner; however, this is insufficient to suppress SAM elevation completely in old flies. Overexpression of gnmt suppresses this age-dependent SAM increase and extends longevity. Pro-longevity regimens, such as dietary restriction or reduced insulin signalling, attenuate the age-dependent SAM increase, and rely at least partially on Gnmt function to exert their lifespan-extending effect in Drosophila. Our study suggests that regulation of SAM levels by Gnmt is a key component of lifespan extension.
Similar content being viewed by others
Introduction
The most successful regimen to delay ageing and extend longevity is dietary restriction (DR), which was shown to increase lifespan from yeast to mammals1. However, a total reduction in calories may not be required, as reductions in specific macromolecules, such as amino acids or proteins, alone can also have an effect on organismal lifespan. In particular, methionine restriction (MR) has been shown to be sufficient for lifespan extension in yeast, worms, flies and rodents2,3,4,5. The evolutionally conserved enzyme methionine adenosyltransferase (MAT), also known as S-adenosyl-methionine synthase (Sams), first converts methionine into S-adenosyl-methionine, or SAM, a versatile methyl donor required for almost all methyltransferase (MTase) activity. Since various MTases catalyse the transfer of methyl groups to nucleic acids, proteins and metabolites, changes in SAM levels can result in altered epigenetic regulation, lipid metabolism or protein function6. Moreover, downstream metabolites of SAM include polyamines and cysteine, both of which are related to longevity by inducing autophagy7 and conferring oxidative stress resistance, respectively8. Therefore, SAM and/or its downstream metabolites, rather than methionine, seems to be the main effector of the MR-dependent increase in lifespan. Indeed, knockdown of sams1 in Caenorhabditis elegans, which results in decreased SAM, extends longevity, possibly through a reduction in protein translation9,10. However, loss of MAT1A function in mice induced liver damage, which led to steatosis and eventually developed into hepatocellular carcinoma6. Human patients with chronic liver disease are reported to have decreased capacity to synthesize SAM11, suggesting that a reduction in SAM synthesis is harmful in humans.
The goal of our study was to determine whether alteration of SAM metabolism resulted in positive or negative effects on the Drosophila lifespan. We found that glycine N-methyltransferase (Gnmt) is a predominant regulator of SAM levels in Drosophila. Overexpressing Gnmt could rescue the age-dependent SAM increases and extend Drosophila lifespan. Our data demonstrate that enhanced SAM catabolism by Gnmt is a key factor for lifespan control.
Results
Knockdown of SAM synthase shortened Drosophila lifespan
Drosophila melanogaster has a single SAM synthase (sams) (Fig. 1a). We first knocked down sams to test whether it increases the lifespan in Drosophila, as reported in C. elegans. UAS-sams-RNA interference (RNAi) crossed with an ubiquitous driver, da-Gal4, showed developmental lethality, indicating that sams is an essential gene for proper development. Next, we checked the lifespan of male flies with adult-specific knockdown of sams by TubGS-Gal4, which is the RU486-inducible binary expression system in the whole body. We were able to bypass the developmental lethality of sams-RNAi, and thus, we analysed the adult lifespan in the presence or absence of RU486. In contrast to sams-RNAi in C. elegans, we observed a significantly shortened lifespan in Drosophila (Fig. 1b).
(a) Schematic view of methionine metabolism in Drosophila melanogaster. Gly, glycine; Sar, sarcosine; Met, methionine; SAM, S-adenosyl-methionine; SAH, S-adenosyl-homocysteine; Hcy, homocysteine; Sams, S-adenosyl-methionine synthase; Gnmt, glycine N-methyltransferase. (b) Lifespan analysis of ubiquitous sams-RNAi male flies under 10% SY diet. In total, 200 μM RU486 (RU) is used to knock down sams after adult eclosion in TubGS>sams-RNAi. Statistics, log-rank test, P<0.0001 (N=70 for −RU, N=79 for +RU). (c) Western blot analysis of Sams and Gnmt in day-5 male flies with either sams-RNAi or gnmt-RNAi driven by the fat-body drivers: r4-Gal4 and FB-Gal4, or no driver. + Indicates UAS-only or Gal4-only controls. (d–l) UPLC–MS/MS analysis of SAM, methionine (Met) and sarcosine (Sar) levels in day-5 male flies with either sams-RNAi or gnmt-RNAi driven by the fat-body drivers (FB-Gal4, r4-Gal4) or no driver. + Indicates UAS-only or Gal4-only controls. Error bars represent mean±s.d. (N=4). Statistics, one-way analysis of variance with Bonferroni’s multiple comparison test. *P<0.05, **P<0.01, ***P<0.001 from the biological replicates. NS, not significant.
It was possible that sams-RNAi had tissue-specific negative effects. Because methionine metabolism is active in the liver of mammals, we knocked down sams specifically in the fat body, a counterpart of the liver and white adipose tissue in Drosophila. We compared the lifespan of FB>sams-RNAi flies to UAS-only control flies, as FB-Gal4, but not UAS-sams-RNAi, was backcrossed in this experiment. Both the female and male lifespans were significantly decreased by knocking down sams in fat body (Supplementary Fig. 1a,b). Although this manipulation is not adult specific, as FB-Gal4 is also expressed during the developmental stage, inhibiting SAM synthesis in Drosophila did not demonstrate positive effects on lifespan, raising a question about what causes the different outcomes between flies and worms.
Gnmt is a dominant regulator of systemic SAM levels
We suspected that there were differences in the metabolic regulation of SAM. To find out how SAM metabolism was affected by sams-RNAi, we analysed the metabolites of methionine metabolism by ultra-performance liquid chromatography tandem mass spectrometry (UPLC–MS/MS) (Fig. 1c–l). Surprisingly, sams-RNAi in the fat body using two different fat-body Gal4 drivers (FB-Gal4 and r4-Gal4) did not result in a systemic SAM reduction (Fig. 1d,g), despite the marked decrease in Sams proteins (Fig. 1c). Methionine was drastically elevated, indicating that the synthesis of SAM from methionine was indeed inhibited (Fig. 1e,h). Fruit flies, mice and humans, but not worms, have Gnmt (http://www.genome.jp/kegg/) that catalyses the conversion of glycine to sarcosine (N-methyl-glycine) by methyl group transfer using SAM (Fig. 1a). Gnmt works as a regulator of SAM levels in metabolic organs such as liver in mammals or the fat body in Drosophila6,12. Knockdown of gnmt in fat body led to reduced Gnmt proteins (Fig. 1c), elevated SAM levels (Fig. 1d,g) and decreased Sar levels (Fig. 1f,i), although there is an unexpected slight increase in all three metabolites in negative-control flies (Fig. 1j–l). When methionine was added to food, the increase in SAM levels was limited, whereas the amount of sarcosine was increased in a dose-dependent manner (Supplementary Fig. 1c–e). Conversely, sarcosine levels significantly decreased in sams knockdown male flies (Fig. 1f,i), in contrast to negative-control flies (where they were increased for unknown reasons) (Fig. 1l), indicating that Gnmt maintains SAM levels by reducing SAM consumption. Interestingly, sams-RNAi in the fat body led to a reduction in Gnmt protein (Fig. 1c), which corresponded to a reduced amount of sarcosine, suggesting the existence of transcriptional or post-transcriptional regulation of Gnmt expression for maintaining SAM levels. Considering that sams1-RNAi resulted in a decrease in the amount of SAM in C. elegans13, the buffering of SAM levels by Gnmt may explain the different sams-RNAi phenotype. However, there are other possibilities, including differences in the knockdown efficiency of sams or the number of sams genes (four in C. elegans, but only one in D. melanogaster).
We further analysed how these metabolites are affected by overexpressing either sams or gnmt ubiquitously or in a fat-body-specific manner (Fig. 2a–i; supplementary Fig. 2a). sams overexpression decreased Met and increased SAM. Introducing the R275H mutation, which was observed in several human hypermethioninemia patients who had a dominant MAT1AR264H mutation14, led to increased Met without affecting SAM levels. This result resembled the sams-RNAi phenotype. MAT1A in mammals forms dimers (called MAT III) or tetramers (called MAT I). R264 is essential for dimerization, and the R264/R264H dimer is enzymatically inactive14. Thus, we considered that SamsR275H may behave as a dominant-negative form in Drosophila as well. On the other hand, gnmt overexpression decreased SAM levels, although subtly, as well as methionine levels. The reason methionine was also reduced in gnmt-overexpressing male flies was unknown; however, we thought that the reduction in SAM accelerated SAM synthesis from methionine as a feedback mechanism for maintaining SAM levels. We also established a mutant version of a gnmt overexpression line by introducing a serine 145 to alanine substitution. Since S145A is located at the SAM-binding site of Gnmt15, we speculated that this mutation might result in reduced enzymatic Gnmt function. Indeed, the effect of gnmtS145A overexpression on SAM and methionine was attenuated, although it was not complete (Fig. 2a–i; supplementary Fig. 2b–d).
(a–i) UPLC–MS/MS analysis of SAM, Met and Sar levels in day-5 male flies with sams, samsR275H, gnmt and gnmtS145A overexpression by ubiquitous (da-Gal4) or fat-body (r4-Gal4) drivers or no driver. + Indicates UAS-only or Gal4-only controls. Statistics, two-tailed unpaired t-test. Error bars represent mean±s.d. (N=5 for da-Gal4, N=3 for r4-Gal4, N=4 for no-driver control). *P<0.05, **P<0.01, ***P<0.001 from the biological replicates. NS, not significant.
Gnmt overexpression increased longevity
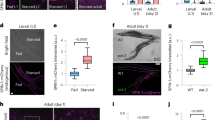
We then assessed whether SAM reduction in gnmt-overexpressing flies had a positive impact on lifespan. When gnmt was overexpressed using TubGS-Gal4, we observed a slight but statistically significant increase in male lifespan (Fig. 3a,b). Several enzymes, including Gnmt, have functions other than enzymatic activity16, making us question whether Gnmt-induced longevity is dependent on its enzymatic activity. Overexpression of gnmtS145A by TubGS-Gal4 did not show any significant effect on lifespan, confirming that enhancing enzymatic activity of Gnmt is necessary for lifespan extension (Fig. 3a,b). Unexpectedly, we did not observe the lifespan increase in female flies when we compared lifespans of gnmt with those of gnmt+RU (Supplementary Fig. 3a,b). However, there was a significant increase in gnmt when compared with gnmtS145A, even in the absence of RU. It is possible that leaky expression of the TubGS driver was enough to extend lifespan in females, or that the position (attP40) effect of UAS-gnmt could have different effects on males and females. Therefore, we also checked the effect of Gnmt overexpression on lifespan using a different gnmt overexpression line, which was inserted at the attP2 site (gnmt-2). In line with this, we observed a significant increase in male and female lifespans (Fig. 3c,d), suggesting that Gnmt overexpression can be beneficial for both sexes.
(a) Lifespan analysis of Gnmt-overexpressing male flies by TubGS-Gal4. Statistics, log-rank test, gnmt versus gnmt+RU, P<0.0001 (N=117 for gnmt, N=118 for gnmt+RU). gnmtS145A versus gnmtS145A+RU, P=0.7732 (N=111 for gnmtS145A, N=113 for gnmtS145A+RU). (b,d,f,j,l) Average lifespan. One-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test was used for statistical analysis. (c) Lifespan analysis of gnmt-overexpressing male and female flies by TubGS-Gal4. UAS-gnmt integrated on attP2 site is used. Statistics, log-rank test, gnmt-2 male versus gnmt-2+RU male, P<0.0001 (N=116 for each). gnmt-2 female versus gnmt-2+RU female, P<0.0001 (N=116 for each). (e) Lifespan analysis of male flies overexpressing InRDN (dominant-negative form of the insulin receptor), gnmt-RNAish or both. Statistics, log-rank test, InRDN versus InRDN+RU, P<0.0001 (N=158 for InRDN, N=154 for InRDN+RU). gnmt-RNAish versus gnmt-RNAish+RU, P=0.0478 (N=80 for both). InRDN, gnmt-RNAish versus InRDN, gnmt-RNAish+RU, P<0.0001 (N=142 for InRDN, gnmt-RNAish, N=154 for InRDN, gnmt-RNAish+RU). (g) qRT–PCR analysis of InR in day-7 adult male flies treated RU486 for 5 days. Error bars represent mean±s.e.m. (N=4). Statistics, one–way ANOVA with Bonferroni’s multiple comparison test. (h) Lifespan analysis of male flies overexpressing sams. Statistics, log-rank test, sams versus sams+RU, P=0.6381 (N=135 for sams, N=143 for sams+RU). Inset, average lifespan. (i) Lifespan analysis of male gnmtMi flies under dietary restriction. Statistics, log-rank test, yw 20 versus 5%, P<0.0001 (N=134 for 20%, N=132 for 5%). gnmtMi 20 versus 5%, P=0.5904 (N=133 for 20%, 129 for 5%). (k) Lifespan analysis of female gnmtMi flies under dietary restriction. Statistics, log-rank test, yw 20 versus 5%, P<0.0001 (N=140 for 20%, N=133 for 5%). gnmtMi 20 versus 5%, P=0.9107 (N=134 for 20%, 136 for 5%). *P<0.05, **P<0.01, ***P<0.001 from the biological replicates. NS, not significant.
Gnmt is essential for lifespan-extending regimens
Genetic and pharmacological interventions targeting key metabolic pathways such as insulin/IGF-1 signalling (IIS) or the target of rapamycin (TOR) pathway also increase organismal lifespan1. Despite accumulating genetic studies using model organisms, our knowledge of the mechanisms underlying lifespan extension by DR or IIS/TOR inhibition is not complete. For example, inhibiting the IIS pathway exerts its effect on longevity through the transcription factor FoxO. However, the precise molecular targets and mechanism of FoxO-dependent lifespan increase have not been fully revealed. Interestingly, studies in C. elegans indicated sams-1 expression is negatively regulated by daf-16, an orthologue of FoxO, implying that activation of FoxO under reduced IIS/TOR signalling or DR can extend longevity through repression of sams-1 mesenger RNA9,17,18,19. In Drosophila, dFoxO transcriptionally induces gnmt12, but it did not repress sams at least at the whole-body level (Supplementary Fig. 3c), implying that Gnmt may contribute to IIS/FoxO-dependent lifespan extension. We observed in our experimental conditions that adult-specific overexpression of InRDN in male flies significantly increased lifespan (Fig. 3e,f). As expected, we showed that knockdown of gnmt partially attenuated the lifespan extension of TubGS>InRDN (Fig. 3e,f), suggesting that Gnmt mediates lifespan extension under reduced IIS activity. This was not due to the dilution of Gal4 by introducing two UAS lines simultaneously, as we observed the same degree of InRDN induction by RU treatment in both lines (Fig. 3g). Interestingly, gnmt-RNAi alone did not affect lifespan, indicating that increases in SAM do not have a negative impact on the lifespan (Fig. 3e,f). This was also supported by the data that sams overexpression in male flies did not affect the lifespan (Fig. 3h).
We also tested whether DR-induced longevity requires Gnmt. We used 20% SY diet as nutrient-rich food and 5% SY diet as DR diet. When compared with nutrient-rich condition, DR significantly extended the lifespans of both male and female flies in our lab conditions (Fig. 3i–l). Then, we subjected the gnmtMi loss-of-function mutant, which is the protein null mutant that we characterized previously and in which neither Gnmt protein nor sarcosine could be detected12, to DR lifespan analysis. Although gnmtMi has a different genetic background since it showed lethality when backcrossed to w1118, we found that DR-induced longevity was not observed in gnmtMi mutant (Fig. 3i–l), suggesting the possibility that Gnmt-dependent SAM catabolism mediates DR longevity. In addition, gnmt expression is positively regulated by the oxidative and xenobiotic stress-responsive factor CncC/Nrf2 (Supplementary Fig. 3d–h)20, which is also suggested as a mediator of DR longevity21, indicating that Gnmt is a common downstream target for longevity pathways.
SAM levels increase during ageing despite Gnmt induction
Although Gnmt overexpression extended longevity, and Gnmt is required for DR-induced longevity, it is still unknown whether Gnmt activity and SAM metabolism changes during physiological ageing. We quantified Met and SAM in two different wild-type strains analysing both young and aged male flies. Met levels decreased during ageing, whereas SAM levels increased (Fig. 4a–d). We also found that the amount of sarcosine decreased during ageing (Fig. 4e), suggesting Gnmt activity to buffer SAM declines in an age-dependent manner. However, the expression of Gnmt was induced in aged male flies, as determined by quantitative real-time PCR (qRT–PCR) and western blotting using whole-body homogenates (Fig. 4f,g; Supplementary Fig. 4a). Sarcosine levels are negatively regulated by sarcosine dehydrogenase (sardh)12, and Sardh was also induced transcriptionally during ageing (Fig. 4f,h). Interestingly, when sardh expression was knocked down, sarcosine levels were high in young male flies and further induced by ageing (Fig. 4i), suggesting that total Gnmt activity is indeed increased during ageing. Gnmt was increased in the fat body, because fat-body-specific knockdown of gnmt abrogated elevation of Gnmt in whole-body homogenates (Supplementary Fig. 4b). This increase was dependent on dFoxO (Fig. 4j; Supplementary Fig. 4c). Induction of Gnmt expression during ageing might be an adaptive response against an increase in SAM levels. Indeed, when dFoxO was knocked down in the fat body, SAM levels in aged male flies were further elevated (Fig. 4k). Therefore, we concluded that the dFoxO–Gnmt pathway is activated, but not sufficiently for complete suppression of the SAM increase in aged flies. Importantly, overexpression of gnmt, but not gnmtS145A, inhibited the age-dependent SAM increase (Fig. 4k; Supplementary Fig. 4d,e), indicating that Gnmt-induced lifespan extension is caused by suppression of the age-dependent increase in SAM.
(a–d) UPLC–MS/MS analysis of SAM and Met levels in wild-type male flies during ageing. Error bars represent mean±s.d. (N=4). Statistics, one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. (e) UPLC–MS/MS analysis of sarcosine levels in male w1118 during ageing. Error bars represent mean±s.d. (N=3). Statistics, one-way ANOVA with Bonferroni’s multiple comparison test. (f) Western blot analysis of Sardh and Gnmt during ageing in male w1118. (g,h) qRT–PCR analysis of gnmt and sardh in young (1 week old) and old (7 weeks old) wild-type male flies. Error bars represent mean±s.e.m. (N=5 for 1 w w1118, N=6 for 7 w w1118, N=3 for Canton S). Statistics, two-tailed unpaired t-test 1 versus 7 w. (i) UPLC–MS/MS analysis of sarcosine levels in young and old male flies with lacZ-RNAi or sardh-RNAi. Error bars represent mean±s.d. (N=4). Statistics, One-way ANOVA with Bonferroni’s multiple comparison test. (j) Western blot analysis of Gnmt in dFoxO knockdown flies.+ Indicates Gal4-only controls. (k) UPLC–MS/MS analysis of SAM levels in young and old male flies with lacZ-RNAi, dFoxO-RNAi, gnmt or gnmtS145A. Error bars represent mean±s.d. (N=4). Statistics, one-way ANOVA with Bonferroni’s multiple comparison test. w, week. *P<0.05, **P<0.01, ***P<0.001 from the biological replicates.
SAM levels are maintained under pro-longevity regimens
Since Gnmt is required for DR-dependent lifespan extension, we analysed how DR altered systemic SAM levels. We analysed young (1 weeks (w)) and aged (5 w) male flies from two wild-type strains as well as a gnmt mutant strain maintained on a DR- (5% SY) or nutrient-rich (20% SY) diet. Since the 20% SY diet contains much Met, young flies maintained on 20% SY diet showed a slight increase in Met levels; however, changes in SAM levels were not statistically significant (Fig. 5a,b; Supplementary Fig. 5a,b), suggesting that Gnmt buffered SAM increase. Indeed, the lack of regulation by Gnmt resulted in increased SAM in male flies on the 20% SY diet compared with that of files on the 5% SY diet (Fig. 5a). As previously mentioned, Met levels tended to decrease with ageing in all three genotypes, but this phenotype was not affected by the diet (Fig. 5b; Supplementary Fig. 5b). In contrast, an age-dependent increase in SAM levels was suppressed by DR (Fig. 5a; Supplementary Fig. 5a). The gnmt mutants showed high SAM levels in young flies, but this was not further elevated by ageing (Fig. 5a), suggesting that a threshold for SAM increases exists in aged flies.
(a,b) UPLC–MS/MS analysis of SAM and Met levels in yw and gnmtMi young (1 weeks old) or old (5 weeks old) male flies maintained under dietary restriction or a nutrient-rich condition. Error bars represent mean±s.d. (N=3–4). Statistics: one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. (c–e) UPLC–MS/MS analysis of SAM levels in lacZ-, InRDNor TORTED-overexpressing male flies at young (1 week old) or old (5 weeks old) stages. Error bars represent mean±s.d. (N=3–4). Statistics: one-way ANOVA with Bonferroni’s multiple comparison test. (f) Schematic view of the relationship between SAM metabolism and longevity control. Compared with young flies, SAM levels are increased despite Gnmt induction in old flies. Strengthening the dFoxO–Gnmt axis rescues age-dependent SAM increases and extends lifespan. *P<0.05, **P<0.01, ***P<0.001 from the biological replicates. NS, not significant.
In addition to DR, we checked whether reduced IIS activity affected systemic SAM levels by analysing TubGS>InRDN. Compared with control flies (TubGS>lacZ), in which SAM was increased during ageing regardless of RU486 treatment (Fig. 5c), SAM levels were rather significantly decreased in TubGS>InRDN old male flies upon RU486 treatment than that in the control flies (old flies without RU486 or young flies with RU486), although the reason behind the RU486-induced SAM elevation in TubGS>InRDN young flies is unknown (Fig. 5d). Interestingly, an age-dependent SAM increase was also rescued by overexpressing the dominant-negative form of the TOR (TubGS>TORTED) (Fig. 5e). These data suggested that the suppression of SAM increases in aged flies was a common mechanism underlying lifespan extension by DR or reduced IIS/TOR pathway.
Discussion
Our study indicates that the enhancement of SAM catabolism by Gnmt is an essential component for lifespan extension (Fig. 5f). Although Gnmt is transcriptionally induced during ageing at a site downstream of dFoxO activity in the fat body, this seemed to be insufficient to maintain SAM levels in aged flies. The reason behind the increase in SAM during ageing has yet to be elucidated; however, strengthening Gnmt activity attenuates the elevation of SAM and, importantly, extends longevity. Moreover, our data implied that DR and reduced IIS signalling (probably TOR and CncC as well) commonly target SAM metabolism to extend lifespan by inducing Gnmt. In humans, whether SAM levels increase in an age-dependent manner remains unknown, since only a few studies have tested this. However, one report suggested that serum SAM levels were higher in older individuals than in middle-aged individuals, at least in some populations22.
In our experimental conditions, sams-RNAi resulted in shorter lifespans. If present in excess, Met is a toxic compound in Drosophila4. It is therefore possible that hypermethioninemia in sams knockdown flies, the MAT1A knockout mice and patients with MAT1A deficiency causes adverse health effects4,23. However, whether sams1-RNAi in C. elegans results in the accumulation of methionine is unknown. Unexpectedly, loss of Gnmt function and subsequent SAM elevation did not have a negative effect on lifespan. The fact that the correlation of SAM levels and lifespan is not bidirectional implies a threshold in SAM levels that modulate organismal lifespan. One explanation is the biochemical character (for example, Km) of methyltransferases or other enzymes related to SAM-dependent metabolic pathways such as polyamine biosynthesis, methionine salvage pathway or trans-sulfuration pathway (TSP), as excess SAM does not always lead to elevated methylation or increased downstream metabolites.
The fact that Gnmt overexpression increases Drosophila lifespan suggests that decreases in SAM (and Met) and/or increase in SAM catabolites have a positive effect on longevity. For example, the acceleration of SAM catabolism by Gnmt may enhance the TSP, which will increase anti-oxidative capacity by upregulating cysteine, taurine and glutathione synthesis. In addition, TSP is critical for producing hydrogen sulfide, H2S, which is suggested to be the mediator of DR-induced benefits in both hepatic damage from ischaemia/reperfusion in mice and longevity in worms24. A study in Drosophila also suggests that TSP mediates DR-induced longevity25. Therefore, TSP or H2S might represent an underlying mechanism for Gnmt-dependent lifespan extension. In the sams-overexpressing flies in our study, SAM and probably downstream metabolites are increased. However, we did not observe any effect on lifespan in these flies, suggesting that not only enhancing SAM catabolism but also reducing SAM under the threshold is required for lifespan extension. In contrast, Gnmt overexpression reduces SAM and simultaneously enhances the generation of SAH and downstream metabolites. Whether reduction of SAM without enhancing SAM catabolism is sufficient for lifespan extension is not known, although it is suggested by the fact that sams-RNAi in worms can extend lifespan. Since lifespan represents a total sum of both positive and negative effect of different pathways, it is difficult to pinpoint the SAM-related pathway(s) essential for lifespan control, until we elucidate how each component affects lifespan.
It is also possible that SAM amount in host cells is recognized as a hallmark of nutrition availability. Thus, SAM reduction triggers the ‘fasting’ response. For example, in yeast, nutrient poor diet induced autophagy, which was inhibited by methionine at least partially through the regulation of SAM-dependent PP2A methylation by ppm1 methyltransferase26. Autophagy, induced by MR, was reported to be a direct cause of lifespan extension3, suggesting that SAM reduction-induced autophagy extends longevity, although no orthologue of ppm1 is found in Drosophila. SAM-dependent transmethylation, including ribosomal RNA methylation, that affects lifespan through modulating translation27 is another possible connection between SAM and longevity. The exact molecular mechanisms behind the SAM effect on lifespan need to be investigated.
Ames dwarf mice are long-lived mutants that have defects in the production of growth hormone (GH) with consequent reductions in IGF-1 levels. Interestingly, Ames dwarf mice also show elevated GNMT expression and activity in addition to reduced SAM levels in their liver28,29. Administration of GH to Ames dwarf reduced GNMT activity while GH receptor knockout mice showed increased GNMT expression, indicating that GH signalling negatively regulates GNMT30. Although the contribution of GNMT in longevity was not studied, MR did not further extend lifespan in Ames dwarf mice, suggesting that altered methionine metabolism is responsible for longevity in these animals31. gnmt is one of seven genes commonly upregulated under DR (or resveratrol treatment) conditions in flies and mice32, further demonstrating that the positive effects of enhanced gnmt activity on longevity in mammals is conserved.
Methods
Fly stocks
Flies were reared on a standard diet containing 4% cornmeal, 4% baker’s yeast (Oriental Yeast), 10% glucose and 0.8% agar with propionic acid and nipagin at constant 25 °C, 60% humidity under 12–12-h light–dark conditions. For most biochemical analysis other than lifespan study, all flies were collected within 2 days after adult eclosion and maintained for 5 days with free access to food and mating for adult maturation, unless otherwise stated.
w1118, yw and Canton S were utilized as wild-type strains. UAS-gnmt-RNAish were generated in our previous study12. TubGS-Gal4 was kindly provided by S. Pletcher. UAS-CncC was kindly provided by D. Bohmann. r4-Gal4, UAS-InRDN (K1409A), UAS-dFoxO-RNAi (HMS00422) and UAS-dFoxO were obtained from the Bloomington Drosophila stock center. UAS-gnmt-RNAi (v25983), UAS-sams-RNAi (v103143) and UAS-keap1-RNAi (v107052) were obtained from the Vienna Drosophila resource center. da-Gal4, FB-Gal4, Mef2-Gal4, pxn-Gal4, NP1-Gal4, elav-Gal4, UAS-gnmt-RNAi and UAS-lacZ-RNAi were all backcrossed at least six generations into w1118. yw;gnmtMi was maintained on its original genetic background, because it shows lethality when backcrossed onto w1118. Thus, we used the yw line as a control. UAS-gnmt, UAS-gnmtS145A, UAS-sams and UAS-samsR275H were generated as described below. For overexpression of metabolic enzymes in fat body, r4-Gal4 was used because it is stronger than FB-Gal4. For most biochemical analyses of adult male flies, we crossed the same batch of Gal4 virgin females (once mixed after virgin collection, and then separated into each group) with different males including wild-type or lacZ lines as control. UAS-only controls were also analysed for some experiments, which we believe is essential.
Construction and generation of transgenic flies
We established gnmt overexpression lines by phiC31-integrase-mediated transgenesis as follows. For making UAS-gnmt, a PCR-amplified 870-bp fragment of the full-length complementary DNA (cDNA)12 was reinserted into the pUAS-attB vector by EcoRI/XhoI, and it was then inserted into the attP40 site using phiC31-mediated transgenesis (Best Gene). For UAS-gnmtS145A, we subcloned full-length gnmt cDNA once into the pBSSK vector by EcoRI/XhoI and introduced the TCC→GCA mutation by PCR-based site-directed mutagenesis using the following primers: 5′-GGCAACGCATTTGCCCACTTGATGGAC-3′, and 5′-GGCAAATGCGTTGCCCAAGCAAATGAC-3′, followed by reinsertion into the pUAS-attB vector. For UAS-sams, a 1.2-kbp cDNA sams fragment was amplified by PCR from total RNA of adult w1118 using the following primers: 5′-GAATTCATGCCGCAAAAGAC-3′, and 5′-CTCGAGTCAGTTGTCAATCTCC-3′ and cloned into the pT7 vector. sams cDNA was amplified by PCR using pT7-sams as template and the primers: 5′-GAAGATCTATGCCGCAAAAGAC-3′, and 5′-GGGCTCGAGTCAGTTGTCAATCTCC-3′ and subcloned once into the pUAST-3xFlag vector. The vector was then sequenced. Full-length, 1.2-kbp sams cDNA without a Flag tag was digested by Bgl II/XhoI and subcloned into the pUAST-attB vector. For UAS-samsR275H, sams cDNA was subcloned once into the pBSSK vector and a AGA→CAC mutation was introduced by PCR-based site-directed mutagenesis using the following primers: 5′-CTTACAGGACACAAAATCATTGTAGATACT-3′, and 5′-AATGATTTTGTGTCCTGTAAGCCCAGCGTC-3′. Transgenes were inserted into the attP40 site on the second chromosome or attP2 site on the third chromosome using phiC31-mediated transgenesis (Best Gene) and maintained as homozygous lines without crossing with balancer lines.
Fly diet
For DR experiments, we prepared a 20% SY diet and 5% SY diet25. The 20% SY diet contains 20% baker’s yeast (Oriental Yeast), 20% sucrose (Wako), and 1.5% agar (Kishida Chemical) with propionic acid and nipagin. The 5% SY diet contains 5% baker’s yeast (Oriental Yeast), 5% sucrose (Wako) and 1.5% agar (Kishida Chemical) with propionic acid and nipagin. For making these diet, first yeast and sucrose were dissolved in water and boiled for 15 min to kill the living yeast completely. This mixture was added into agar, which was dissolved in water with 10-min heating in the different pot. After that, propionic acid and nipagin were added directly into the diet. For the gene switch (GS) system, 10% SY diet was prepared and RU486 (Sigma) was added into the 10% SY diet at a final concentration of 200 μM to drive gene expression. For the high-Met diet, we added 1, 2 or 5 ml of 100 mM stock methionine solution in a 100-ml standard diet.
Lifespan analysis
To control environmental effects during development, we crossed the same number of female and male flies in the same period on a standard diet. Newly hatched flies were collected within, at most, 2 days, and then they were kept for 2 days under the mixed sex condition. Then, males and females were separated and grouped into 20 flies per vial. Flies were transferred to fresh food every 3 or 4 days, and at that time, we counted the number of dead flies.
Western blotting and quantitative RT–PCR analysis
For western blot analysis, five adult male flies were homogenized in PBS supplemented with 0.1% SDS, 10 mM dithiothreitol and 1 × protease inhibitor cocktail (Roche). Samples were boiled for 5 min at 98 °C after mixing with 6 × Laemmli sample buffer. Whole-body proteins corresponding approximately to 0.15 fly were subjected to SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane. After 30 min blocking with 5% skimmed milk, membranes were incubated with the 1st antibody at 4 °C overnight. Anti-Gnmt antibody (1:3,000) and anti-Sardh antibody (1:1,500) were generated previously12. Anti-Sams antibody (1:1,000) was produced in guinea pigs using a synthetic peptide corresponding to the C terminus 382–400 of Drosophila Sams as an antigen. For loading control, anti-α-tubulin monoclonal antibody (1:3,000, Sigma DM1A) was used. Representative images were shown from at least two independent experiments with reproducible results. Full blot images for main figures are shown in Supplementary Fig. 6. For qRT–PCR, total RNA was purified from five adult males using the Qiazol and RNeasy micro kit (Qiagen). cDNA was made from 100 or 200 ng DNase-treated total RNA by the Takara PrimeScript RT Reagent Kit with gDNA Eraser. Quantitative PCR was performed using Takara Premix Ex Taq II (Tli RNaseH Plus) and the Light Cycler 480 system (Roche). For internal controls, Rpl32 was predominantly used, but RNA pol II was also checked to rule out the possibility that changes in target gene expression resulted from altered expression of internal controls. Primer sequences are available in Supplementary Table 1.
Measurement of metabolites by LC–MS/MS
Methionine, SAM and sarcosine levels were measured by UPLC equipped with tandem MS, TQD (UPLC–MS/MS, Waters)12. Briefly, five adult males were homogenized in 50% methanol, deproteinized by acetonitrile and evaporated completely. Pellets were solubilized in 10 mM HCl followed by filtration using 0.22-μm polyvinylidene difluoride filters (Millipore). Samples were mixed in equal volume of 50 mM Tris-HCl pH 8.8 with 100 μM dithiothreitol for Met and SAM measurements. For the sarcosine measurement, samples were derivatized after filtration by the AccQ-Tag Ultra Derivatization Kit (Waters). Samples were subjected to UPLC system with Acquity UPLC BEH C18 column. Separated solutions were ionized by electrospray ionization in positive-ion mode (ESI+) and detected by TQD with the following m/z transitions: Methionine, 149.98>132.90, SAM, 399.20>250.00 and derivatized sarcosine, 260.00>171.50.
Because absolute metabolite amounts can easily vary between experiments due to technical (for example, machine conditions) as well as biological (for example, season, food, genotype and so on) conditions, we always prepared a control sample in the same analytical round to compare the quantities as ratios relative to the control. To analyse the metabolites precisely, the quality of parents, the developmental environment and sampling time (to avoid circadian changes) were carefully controlled, and each graph was constructed from the same round of analysis.
Additional information
How to cite this article: Obata, F. & Miura, M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat. Commun. 6:8332 doi: 10.1038/ncomms9332 (2015).
References
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span--from yeast to humans. Science 328, 321–326 (2010).
Orentreich, N., Matias, J. R., DeFelice, A. & Zimmerman, J. A. Low methionine ingestion by rats extends life span. J. Nutr. 123, 269–274 (1993).
Ruckenstuhl, C. et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. 10, e1004347 (2014).
Lee, B. C. et al. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 5, 3592 (2014).
Cabreiro, F. et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239 (2013).
Lu, S. C. & Mato, J. M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 92, 1515–1542 (2012).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Ingenbleek, Y. & Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 71, 413–432 (2013).
Hansen, M., Hsu, A.-L., Dillin, A. & Kenyon, C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 1, 119–128 (2005).
Ching, T.-T., Paal, A. B., Mehta, A., Zhong, L. & Hsu, A.-L. drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging Cell 9, 545–557 (2010).
Martínez-Chantar, M. L. et al. Importance of a deficiency in S-adenosyl-L-methionine synthesis in the pathogenesis of liver injury. Am. J. Clin. Nutr. 76, 1177S–1182SS (2002).
Obata, F. et al. Necrosis-driven systemic immune response alters SAM metabolism through the FOXO-GNMT axis. Cell Rep. 7, 821–833 (2014).
Walker, A. K. et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 147, 840–852 (2011).
Chamberlin, M. E., Ubagai, T., Mudd, S. H., Levy, H. L. & Chou, J. Y. Dominant inheritance of isolated hypermethioninemia is associated with a mutation in the human methionine adenosyltransferase 1A gene. Am. J. Hum. Genet. 60, 540–546 (1997).
DebRoy, S. et al. A novel tumor suppressor function of glycine N-methyltransferase is independent of its catalytic activity but requires nuclear localization. PLoS ONE 8, e70062 (2013).
Yen, C.-H., Lin, Y.-T., Chen, H.-L., Chen, S.-Y. & Chen, Y.-M. A. The multi-functional roles of GNMT in toxicology and cancer. Toxicol. Appl. Pharmacol. 266, 67–75 (2013).
Chen, D. et al. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep. 5, 1600–1610 (2013).
Depuydt, G. et al. Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Mol. Cell. Proteomics 12, 3624–3639 (2013).
Dong, M.-Q. et al. Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science 317, 660–663 (2007).
Misra, J. R., Horner, M. A., Lam, G. & Thummel, C. S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 25, 1796–1806 (2011).
Sykiotis, G. P., Habeos, I. G., Samuelson, A. V. & Bohmann, D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care 14, 41–48 (2011).
Inoue-Choi, M. et al. One-carbon metabolism nutrient status and plasma S-adenosylmethionine concentrations in middle-aged and older Chinese in Singapore. Int. J. Mol. Epidemiol. Genet. 3, 160–173 (2012).
Mudd, S. H. Hypermethioninemias of genetic and non-genetic origin: a review. Am. J. Med. Genet. C. Semin. Med. Genet. 157C, 3–32 (2011).
Hine, C. et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 (2014).
Kabil, H., Kabil, O., Banerjee, R., Harshman, L. G. & Pletcher, S. D. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc. Natl Acad. Sci. USA 108, 16831–16836 (2011).
Sutter, B. M., Wu, X., Laxman, S. & Tu, B. P. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell 154, 403–415 (2013).
Schosserer, M. et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat. Commun. 6, 6158 (2015).
Brown-Borg, H. M., Rakoczy, S. G., Sharma, S. & Bartke, A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp. Gerontol. 44, 10–19 (2009).
Uthus, E. O. & Brown-Borg, H. M. Altered methionine metabolism in long living Ames dwarf mice. Exp. Gerontol. 38, 491–498 (2003).
Brown-Borg, H. M., Rakoczy, S. G. & Uthus, E. O. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech. Ageing Dev. 126, 389–398 (2005).
Brown-Borg, H. M. et al. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell 13, 1019–1027 (2014).
Antosh, M., Fox, D., Helfand, S. L., Cooper, L. N. & Neretti, N. New comparative genomics approach reveals a conserved health span signature across species. Aging (Albany. NY) 3, 576–583 (2011).
Acknowledgements
We are grateful to S. Pletcher, D. Bohmann, the Vienna Drosophila Resource Center, and the Bloomington Drosophila Stock Center for Drosophila stocks. We thank Miura’s lab members for discussion and technical assistance. This work was supported by grants from the Japanese Ministry of Education, Science, Sports, Culture and Technology (F.O. and M.M.). and the Japan Society for the Promotion of Science (F.O. and M.M.). This work was also supported by the Basic Research Program of the Japan Science and Technology Agency (to MM).
Author information
Authors and Affiliations
Contributions
F.O. and M.M. designed the study, performed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-6 and Supplementary Table 1. (PDF 478 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Obata, F., Miura, M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat Commun 6, 8332 (2015). https://doi.org/10.1038/ncomms9332
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9332
This article is cited by
-
SAM, SAH and C. elegans longevity: insights from a partial AHCY deficiency model
npj Aging (2023)
-
Time-restricted feeding promotes muscle function through purine cycle and AMPK signaling in Drosophila obesity models
Nature Communications (2023)
-
Early-adult methionine restriction reduces methionine sulfoxide and extends lifespan in Drosophila
Nature Communications (2023)
-
FMO rewires metabolism to promote longevity through tryptophan and one carbon metabolism in C. elegans
Nature Communications (2023)
-
Molecular mechanisms of exceptional lifespan increase of Drosophila melanogaster with different genotypes after combinations of pro-longevity interventions
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.