Abstract
A vast number of epidemiological studies suggest an important, but still controversial, role for obesity and adipose tissue mass in breast cancer risk and an association with tumor phenotype. The main conclusions from these studies raise the possibility that the adipose tissue can act as an effector organ that influences both cancer risk and tumor behavior. Here we also review heterotypic mechanisms in breast-cancer tumorigenesis; these mechanisms involve soluble secreted factors from peritumoral cells, extracellular-matrix components and interactions between stromal cells and tumor cells that create a specific and local peritumoral microenvironment. As a special focus, we discuss the increasing evidence for a role of peritumoral adipose tissue and secreted adipokines (such as adiponectin and leptin) in breast cancer; furthermore, the cellular and molecular basis of the peritumoral 'desmoplastic' tissue reaction observed in breast cancer is reviewed in detail.
Key Points
-
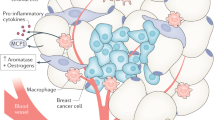
Peritumoral adipose tissue is involved in multiple heterotypic signaling mechanisms, such as secretion of extracellular matrix components and soluble factors from peritumoral adipocytes and interactions between stromal cells and tumor cells
-
Leptin and adiponectin are adiponectins secreted by adipocytes; these adipokines have endocrine and paracrine effects and both regulate mammary epithelial cell behavior and can influence tumor growth and progression
-
The molecular basis of the desmoplastic tissue reaction observed in breast cancer involves tumor necrosis factor, interleukin 11 and matrix metalloproteinase 11, which lead to a local inhibition of adipocyte differentiation
-
Peritumoral adipose tissue and locally produced adipokines could provide future therapeutic targets for breast cancer
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abu-Abid S et al. (2002) Obesity and cancer. J Med 33: 73–86
Key TJ et al. (2001) Energy balance and cancer: the role of sex hormones. Proc Nutr Soc 60: 81–89
Kaur T and Zhang ZF (2005) Obesity, breast cancer and the role of adipocytokines. Asian Pac J Cancer Prev 6: 547–552
Rose DP et al. (2004) Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 5: 153–165
Harvie M et al. (2003) Central obesity and breast cancer risk: a systematic review. Obes Rev 4: 157–173
Daling JR et al. (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92: 720–729
Trentham-Dietz A et al. (1997) Body size and risk of breast cancer. Am J Epidemiol 145: 1011–1019
Galanis DJ et al. (1998) Anthropometric predictors of breast cancer incidence and survival in a multi-ethnic cohort of female residents of Hawaii, United States. Cancer Causes Control 9: 217–224
Kaaks R et al. (2005) Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 97: 755–765
Micheli A et al. (2004) Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer 112: 312–318
Eliassen AH et al. (2006) Endogenous steroid concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst 98: 1406–1415
Wolk A et al. (2001) A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12: 13–21
Bulun SE et al. (1994) Aromatase gene expression in adipose tissue: relationship to breast cancer. J Steroid Biochem Mol Biol 49: 319–326
Schäffler A et al. (2005) Mechanisms of disease: adipocytokines and visceral adipose tissue—emerging role in intestinal and mesenteric diseases. Nat Clin Pract Gastroenterol Hepatol 2: 103–111
Berclaz G et al. (2004) Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol 15: 875–884
Chen DC et al. (2005) Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 237: 109–114
Mantzoros C et al. (2004) Adiponectin and breast cancer risk. J Clin Endocrinol Metab 89: 1102–1107
Miyoshi Y et al. (2003) Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res 9: 5699–5704
Kelesidis I et al. (2006) Adiponectin and cancer: a systematic review. Br J Cancer 94: 1221–1225
Kang JH et al. (2005) Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res 28: 1263–1269
Yamauchi T et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769
Kaser S et al. (2005) Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 54: 117–121
Kadowaki T et al. (2005) Adiponectin and adiponectin receptors. Endocr Rev 26: 439–451
Dieudonne MN et al. (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345: 271–279
Tanko LB et al. (2004) Novel associations between bioavailable estradiol and adipokines in elderly women with different phenotypes of obesity: implications for athergenesis. Circulation 110: 2246–2252
Chen B et al. (2006) Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun 341: 549–556
Fasshauer M et al. (2002) Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 290: 1048–1089
Brown PH and Lippman SM (2000) Chemoprevention of breast cancer. Breast Cancer Res Treat 62: 1–17
Rumi MA et al. (2004) Can PPAR γ ligands be used in cancer therapy? Curr Med Chem Anticancer Agents 4: 465–477
Krzystyniak KL (2002) Current strategies for anticancer chemoprevention and chemoprotection. Acta Pol Pharm 59: 473–478
Burstein HJ et al. (2003) Use of the peroxisome proliferator-activated receptor (PPAR) γ ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat 79: 391–397
Harvie M and Howell A (2006) Energy balance adiposity and breast cancer—energy restriction strategies for breast cancer prevention. Obes Rev 7: 33–47
Surmacz E (2006) Leptin and cancer. J Cell Physiol 207: 12–22
Neville MC et al. (2002) Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7: 49–66
Goodwin PJ et al. (2005) Is leptin a mediator of adverse prognostic effects of obesity in breast cancer? J Clin Oncol 23: 6037–6042
Woo HY et al. (2006) Relationships among serum leptin, leptin receptor gene polymorphisms, and breast cancer in Korea. Cancer Lett 237: 137–142
Snoussi K et al. (2006) Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer 6: 38
Comings DE et al. (2003) A multigene test for the risk of sporadic breast carcinoma. Cancer 97: 2160–2170
Miyoshi Y et al. (2006) High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer 118: 1414–1419
Ishikawa M et al. (2004) Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res 10: 4325–4331
Garofalo C et al. (2006) Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res 12: 1447–1453
Lolmede K et al. (2003) Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord 27: 1187–1195
Machinal F et al. (1999) In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology 140: 1567–1574
Garofalo C et al. (2004) Leptin interferes with the effects of the antiestrogen ICI 182,780 in MCF-7 breast cancer cells. Clin Cancer Res 10: 6466–6475
Yin N et al. (2004) Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res 64: 5870–5875
Catalano S et al. (2004) Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem 279: 19908–19915
Attoub S et al. (2000) Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J 14: 2329–2338
Iyengar P et al. (2003) Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene 22: 6408–6423
Wiseman BS and Werb Z (2002) Stromal effects on mammary gland development and breast cancer. Science 296: 1046–1049
Celis JE et al. (2005) Identification of extracellular and intracellular signaling components of the mammary adipose tissue and its interstitial fluid in high risk breast cancer patients: toward dissecting the molecular circuitry of epithelial-adipocyte stromal cell interactions. Mol Cell Proteomics 4: 492–522
Manabe Y et al. (2003) Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol 201: 221–228
Iyengar P et al. (2005) Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest 115: 1163–1176
Andarawewa KL et al. (2005) Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell–adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res 65: 10862–10871
Deb S et al. (2004) Estrogen regulates expression of tumor necrosis factor receptors in breast adipose fibroblasts. J Clin Endocrinol Metab 89: 4018–4024
Meng L et al. (2001) Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ: mechanism of desmoplastic reaction. Cancer Res 61: 2250–2255
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schäffler, A., Schölmerich, J. & Buechler, C. Mechanisms of Disease: adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Rev Endocrinol 3, 345–354 (2007). https://doi.org/10.1038/ncpendmet0456
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0456
This article is cited by
-
Adipose Stroma Accelerates the Invasion and Escape of Human Breast Cancer Cells from an Engineered Microtumor
Cellular and Molecular Bioengineering (2022)
-
The prognostic outcome of ‘type 2 diabetes mellitus and breast cancer’ association pivots on hypoxia-hyperglycemia axis
Cancer Cell International (2021)
-
Outcomes following high- versus low-volume fat transfer following breast reconstruction and conservation—the Canniesburn Experience
European Journal of Plastic Surgery (2020)
-
Inflammation related miRNAs as an important player between obesity and cancers
Journal of Diabetes & Metabolic Disorders (2019)
-
Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells
Scientific Reports (2018)