Abstract
Minimal hepatic encephalopathy (MHE), formerly known as subclinical hepatic encephalopathy, is the mild cognitive impairment commonly seen in patients who have cirrhosis. Current understanding suggests that MHE forms part of the spectrum of hepatic encephalopathy, although this remains to be proven. Although traditionally viewed as having negligible clinical significance, MHE has a significant impact on quality of life. MHE often goes undiagnosed because in many patients there is no evidence of clinically overt signs of impaired cognition. In addition, the diagnostic criteria for MHE have not been standardized, which means that the exact characteristics of MHE remain in question. This Review focuses on the pathogenesis and neuropsychological findings (incorporating neuroimaging) of MHE, as well as the effect of MHE on quality of life and survival, and developments in treatment.
Key Points
-
Minimal hepatic encephalopathy (MHE), formerly known as subclinical hepatic encephalopathy, is the mild cognitive impairment commonly seen in patients who have cirrhosis
-
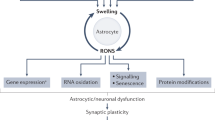
MHE is currently considered to be one end of the clinical spectrum of hepatic encephalopathy (a complex neuropsychiatric disorder), rather than a separate phenomenon; although the pathophysiology of hepatic encephalopathy is still being elucidated, it is known to be linked to hyperammonemia, impaired neurotransmission and cerebral metabolism
-
MHE can have adverse effects on quality of life (e.g. performance in the workplace and driving) and survival, but remains undiagnosed in many patients because they have no clinically overt signs of impaired cognition
-
The diagnostic criteria for MHE have not been standardized but rest on the patient's history, a careful physical examination, the exclusion of other neurological disorders, and the performance of neuropsychological and neurophysiological tests
-
The most established treatment for hepatic encephalopathy is lactulose, but its efficacy has never been evaluated in a placebo-controlled randomized fashion; preliminary data suggest that the use of antioxidants or antibiotics as a potential treatment for MHE warrants further study
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferenci P et al. (2002) Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35: 716–721
Das A et al. (2001) Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol 16: 531–535
Romero-Gomez M et al. (2001) Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 96: 2718–2723
Takano T et al. (2006) Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–270
Olde Damink S et al. (2002) Interorgan ammonia and amino acids metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 36: 1163–1171
Lockwood AH et al. (1991) Altered cerebral blood flow and glucose metabolism in patients with liver disease and minimal encephalopathy. J Cereb Blood Flow Metab 11: 331–336
Lockwood AH et al. (1991) Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 11: 337–341
Guevara M et al. (1998) Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatology 28: 39–44
Barnes EM Jr et al. (1983) Role of glutamine synthetase in the uptake and metabolism of methylammonium by Azotobacter vinelandii. J Bacteriol 156: 752–757
Jayakumar A and Barnes EM Jr (1984) The role of glutamine in regulation of ammonium transport in Azotobacter vinelandii. Arch Biochem Biophys 231: 95–101
Jayakumar A et al. (1986) Role of the Escherichia coli glnALG operon in regulation of ammonium transport. J Bacteriol 166: 281–284
Jayakumar A et al. (1987) Feedback inhibition of ammonium (methylammonium) ion transport in Escherichia coli by glutamine and glutamine analogs. J Bacteriol 169: 553–557
Jayakumar A et al. (1989) Isolation of an ammonium or methylammonium ion transport mutant of Escherichia coli and complementation by the cloned gene. J Bacteriol 171: 996–1001
Bai G et al. (2001) Ammonia induces the mitochondrial permeability transition in primary cultures of rat astrocytes. J Neurosci Res 66: 981–991
Rama Rao KV et al. (2003) Ammonia neurotoxicity: role of the mitochondrial permeability transition. Metab Brain Dis 18: 113–127
Rama Rao KV et al. (2003) Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem Int 43: 517–523
Jayakumar AR et al. (2004) Glutamine-induced free radical production in cultured astrocytes. Glia 46: 296–301
Norenberg MD et al. (2004) Oxidative stress in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 19: 313–329
Norenberg MD et al. (2004) Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr 36: 303–307
Norenberg MD et al. (2005) Mechanisms of ammonia-induced astrocyte swelling. Metab Brain Dis 20: 303–318
Rama Rao KV et al. (2005) Differential response of glutamine in cultured neurons and astrocytes. J Neurosci Res 79: 193–199
Jayakumar AR et al. (2006) Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int 48: 623–628
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65: 1–105
Chan N and Butterworth R (2006) Glutamatergic synaptic regulation deficit in liver failure: a review of molecular mechanisms. In Hepatic Encephalopathy and Nitrogen Metabolism, 160–170 (Eds Haussinger. et al.) Düsseldorf: Springer
Vaquero J and Butterworth RF (2006) The brain glutamate system in liver failure. J Neurochem 98: 661–669
Monfort P et al. (2005) Molecular mechanisms of the alterations in NMDA receptor-dependent long-term potentiation in hyperammonemia. Metab Brain Dis 20: 265–274
Corbalan R et al. (2002) Altered modulation of soluble guanylate cyclase in lymphocytes from patients with liver disease. J Mol Med 80: 117–123
Josephs KA et al. (2005) Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology 64: 2033–2039
Klos KJ et al. (2006) Brain metal concentrations in chronic liver failure patients with pallidal T1 MRI hyperintensity. Neurology 67: 1984–1989
Pujol A et al. (1993) Hyperintense globus pallidus on T1-weighted MRI in cirrhotic patients is associated with severity of liver failure. Neurology 43: 65–69
Lazeyras F et al. (2002) Persistence of mild parkinsonism 4 months after liver transplantation in patients with preoperative minimal hepatic encephalopathy: a study on neuroradiological and blood manganese changes. Transpl Int 15: 188–195
Shawcross DL et al. (2004) Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 40: 247–254
Wright GAK et al. (2006) The role of inflammation in hepatic encephalopathy. In Hepatic Encephalopathy and Nitrogen Metabolism, 229–242 (Eds Haussinger D. et al.) Dordrecht: Springer
Minguez B et al. (2006) Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatology 43: 707–714
Schomerus H and Hamster W (2001) Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis 16: 37–41
Weissenborn K et al. (2001) Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34: 768–773
Weissenborn K et al. (1990) Neurophysiological assessment of early hepatic encephalopathy. Electroencephalogr Clin Neurophysiol 75: 289–295
Kulisevsky J et al. (1992) Pallidal hyperintensity on magnetic resonance imaging in cirrhotic patients: clinical correlations. Hepatology 16: 1382–1388
Lezak MD (1996) Neuropsychological Assessment. New York: Oxford University Press
McCrea M et al. (1996) Neuropsychological characterization and detection of subclinical hepatic encephalopathy. Arch Neurol 53: 758–763
Folstein MF et al. (1975) Mini-Mental State: a practical method for grading the state of patients for the clinician, Journal of Psychiatric Research 12: 189–198
Tarter RE et al. (1987) Neurobehavioral correlates of cholestatic and hepatocellular disease: differentiation according to disease specific characteristics and severity of the identified cerebral dysfunction. Int J Neurosci 32: 901–910
Tarter RE et al. (1984) Nonalcoholic cirrhosis associated with neuropsychological dysfunction in the absence of overt evidence of hepatic encephalopathy. Gastroenterology 86: 1421–1427
Howieson DB and Lezak MD (2002) The neuropsychological evaluation. In Textbook of Neuropsychiatry and Clinical Neurosciences, 217–244 (Eds Yudofsky S and Hales RE) Washington, DC: American Psychiatric Publishing
Joebges EM et al. (2003) Bradykinesia in minimal hepatic encephalopathy is due to disturbances in movement initiation. J Hepatol 38: 273–280
Weissenborn K et al. (2005) Neurological and neuropsychiatric syndromes associated with liver disease. AIDS 19 (Suppl 3): S93–S98
Ortiz M et al. (2006) Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol 44: 104–110
Romero-Gomez M et al. (2007) Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology 45: 879–885
Bajaj JS et al. (2007) Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol 102: 754–760
Rodriguez G et al. (1987) Reduction of cerebral blood flow in subclinical hepatic encephalopathy and its correlation with plasma-free tryptophan. J Cereb Blood Flow Metab 7: 768–772
O'Carroll RE et al. (1991) Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet 337: 1250–1253
Trzepacz PT et al. (1994) SPECT scan and cognitive findings in subclinical hepatic encephalopathy. J Neuropsychiatry Clin Neurosci 6: 170–175
Catafau AM et al. (2000) Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J Nucl Med 41: 405–410
Morgan MY (1998) Cerebral magnetic resonance imaging in patients with chronic liver disease. Metab Brain Dis 13: 273–290
Malecki EA et al. (1999) Iron and manganese homeostasis in chronic liver disease: relationship to pallidal T1-weighted magnetic resonance signal hyperintensity. Neurotoxicology 20: 647–652
Naegele T et al. (2000) MR imaging and (1)H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology 216: 683–691
Weissenborn K et al. (2007) Correlations between MRS alterations and cerebral ammonia and glucose metabolism in cirrhotic patients with and without hepatic encephalopathy. Gut [doi:10.1136/gut.2006.110569]
Huda A et al. (1998) Clinical correlation of neuropsychological tests with 1H magnetic resonance spectroscopy in hepatic encephalopathy. Psychosom Med 60: 550–556
Lockwood AH et al. (1993) Positron-emission tomographic localization of abnormalities of brain metabolism in patients with minimal hepatic encephalopathy. Hepatology 18: 1061–1068
Lockwood AH et al. (2002) Correlations between cerebral glucose metabolism and neuropsychological test performance in nonalcoholic cirrhotics. Metab Brain Dis 17: 29–40
Kale RA et al. (2006) Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology 43: 698–706
Younossi Z et al. (2001) Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol 96: 579–583
Groeneweg M et al. (1998) Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 28: 45–49
Groeneweg M et al. (2000) Screening of subclinical hepatic encephalopathy. J Hepatol 32: 748–753
Marchesini G et al. (2001) Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 120: 170–178
Watanabe A et al. (1995) Evaluation of neuropsychological function in patients with liver cirrhosis with special reference to their driving ability. Metab Brain Dis 10: 239–248
Nyberg S et al. (2006) Early experience with HEADS (hepatic encephalopathy assessment driving simulator) Advances in Transportation Studies: an International Journal: 2006 special issue, 5–15
Wein C et al. (2004) Minimal hepatic encephalopathy impairs fitness to drive. Hepatology 39: 739–745
Sanyal AJ et al. (2006) Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 43: 682–689
Stewart C et al. (2007) Hepatic encephalopathy as a predictor of survival in patients with end stage liver disease. Liver Transplantation 13: 1366–1371
D'Amico G et al. (2006) Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 44: 217–231
Abraldes JG et al. (2003) Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 37: 902–908
Merkel C et al. (1989) Prognostic indicators of survival in patients with cirrhosis and esophageal varices, without previous bleeding. Am J Gastroenterol 84: 717–722
Bustamante J et al. (1999) Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 30: 890–895
Romero-Gomez M et al. (2004) Prognostic value of altered oral glutamine challenge in patients with minimal hepatic encephalopathy. Hepatology 39: 939–943
Quero JC and Schalm SW (1996) Subclinical hepatic encephalopathy. Semin Liver Dis 16: 321–328
Watanabe A et al. (1997) Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology 26: 1410–1414
Liu Q et al. (2004) Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 39: 1441–1449
Mattarozzi K et al. (2004) Minimal hepatic encephalopathy: longitudinal effects of liver transplantation. Arch Neurol 61: 242–247
Mechtcheriakov S et al. (2004) Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 10: 77–83
Hockerstedt K et al. (1992) Encephalopathy and neuropathy in end-stage liver disease before and after liver transplantation. J Hepatol 16: 31–37
Prasad S et al. (2007) Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45: 549–559
Steinbrenner H et al. (2006) Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med 40: 1513–1523
Rothstein JD et al. (2005) Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433: 73–77
Acknowledgements
CAS has received financial support from the NIH (grant K23DK60018) and the Fiterman Foundation, Mayo Clinic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Stewart, C., Smith, G. Minimal hepatic encephalopathy. Nat Rev Gastroenterol Hepatol 4, 677–685 (2007). https://doi.org/10.1038/ncpgasthep0999
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep0999
This article is cited by
-
Cognitive impairment in recipients of liver transplantation and relation to hepatic encephalopathy
Middle East Current Psychiatry (2022)
-
Hyperammonemia in Inherited Metabolic Diseases
Cellular and Molecular Neurobiology (2022)
-
Predictive models of minimal hepatic encephalopathy for cirrhotic patients based on large-scale brain intrinsic connectivity networks
Scientific Reports (2017)
-
Impact of depressive symptoms and hepatic encephalopathy on health-related quality of life in cirrhotic hepatitis C patients
Metabolic Brain Disease (2016)
-
Using saccades to diagnose covert hepatic encephalopathy
Metabolic Brain Disease (2015)