Abstract
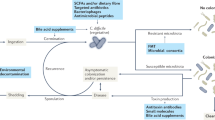
In the past, Clostridium difficile-associated disease (CDAD) was thought of mainly as a nosocomial disease associated with the use of broad-spectrum antibiotics, but its epidemiology seems to be changing. Since 2002, outbreaks of severe CDAD associated with increased mortality and reduced effectiveness of treatment with metronidazole have focused attention on this challenging pathogen. A fluoroquinolone-resistant strain of C. difficile (BI/NAP1/027) has been predominantly associated with these outbreaks. Changes in the epidemiology of CDAD include the emergence of new at-risk populations and the increased incidence of the disease. Infection control programs and more effective treatments offer hope that future outbreaks of CDAD can be controlled.
Key Points
-
The incidence of health-care-associated C. difficile-associated disease (CDAD) is rising
-
Recent outbreaks of CDAD have been characterized by severe disease and increased mortality
-
Atypical hypervirulent BI/NAP1/027 is resistant to fluoroquinolones, produces more toxin and carries the gene encoding binary toxin
-
CDAD might be underdiagnosed if only one stool toxin assay is performed or if atypical at-risk patients are not assayed
-
Control of health-care-associated CDAD can involve a range of strategies
-
Vancomycin and metronidazole remain the mainstays of treatment for CDAD, but newer investigational therapies hold promise
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Berrington A et al. (2004) National Clostridium difficile standards group: report to the Department of Health. J Hosp Infect 56: 1–38
McFarland LV et al. (2007) Implications of the changing face of Clostridium difficile disease for health care practitioners. Am J Infect Control 35: 237–253
Cloud J and Kelly CP (2007) Update on Clostridium difficile associated disease. Curr Opin Gastroenterol 23: 4–9
McDonald LC et al. (2005) An epidemic, toxin gene–variant strain of Clostridium difficile. N Engl J Med 353: 2433–2441
McDonald LC et al. (2006) Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 12: 409–415
Loo VG et al. (2005) A predominantly clonal multi-institutional outbreak of Clostridium-difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 353: 2442–2449
McFarland LV et al. (1989) Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 320: 204–210
McMaster-Baxter NL and Musher DM (2007) Clostridium difficile: recent epidemiologic findings and advances in therapy. Pharmacotherapy 27: 1029–1039
Rupnik M et al. (2005) Revised nomenclature of Clostridium difficile toxins and associated genes. J Med Microbiol 54: 113–117
Geric B et al. (2004) Distribution of Clostridium difficile variant toxinotypes and strains with binary toxin genes among clinical isolates in an American hospital. J Med Microbiol 53: 887–894
Goncalves C et al. (2004) Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile. J Clin Microbiol 42: 1933–1939
McDonald LC et al.; Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 28: 140–145
Rupnik M et al. (2003) New types of toxin A-negative, toxin B-positive strains among Clostridium difficile isolates from Asia. J Clin Microbiol 41: 1118–1125
Delmee M et al. (2005) Laboratory diagnosis of Clostridium difficile-associated diarrhoea: a plea for culture. J Med Microbiol 54: 187–191
Gerding DN (2007) New definitions will help, but cultures are critical for resolving unanswered questions about Clostridium difficile. Infect Control Hosp Epidemiol 28: 113–115
Cookson B (2007) Hypervirulent strains of Clostridium difficile. Postgrad Med J 83: 291–295
Pepin J et al. (2004) Clostridium difficile-associated diarrhea in a region of Québec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171: 466–472
Pepin J et al. (2005) Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173: 1037–1042
MacCannell DR et al. (2006) Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from Eastern and Western Canada. J Clin Microbiol 44: 2147–2152
Warny M et al. (2005) Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366: 1079–1084
Kuijper EJ et al. (2007) Clostridium difficile: changing epidemiology and new treatment options. Curr Opin Infect Dis 20: 376–383
Hubert B et al. (2007) A portrait of the geographic dissemination of the Clostridium difficile North American pulsed-field type 1 strain and the epidemiology of C. difficile-associated disease in Québec. Clin Infect Dis 44: 238–244
Musher DM et al. (2006) Epidemic Clostridium difficile. N Engl J Med 354: 1199–1203
Kuijper EJ et al. (2007) Update of Clostridium difficile-associated disease due to PCR ribotype 027 in Europe. Euro Surveill 12: E1–E2
van Steenbergen J et al. (2005) Isolation of Clostridium difficile ribotype 027, toxinotype III in the Netherlands after increase in C. difficile-associated diarrhoea. Euro Surveill 10: E050714.1
Kuijper EJ et al.; ESCMID Study Group for Clostridium difficile; EU Member States; European Centre for Disease Prevention and Control (2006) Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect 12 (Suppl 6): S2–S18
Delmee M et al. (2006) Epidemiology of Clostridium difficile toxinotype III, PCR-ribotype 027 associated disease in Belgium, 2006. Euro Surveill 11: E060914.2
Kato H et al. (2007) First isolation of Clostridium difficile 027 in Japan. Euro Surveill 12: E070111.3
Tachon M et al. (2006) First cluster of C. difficile toxinotype III, PCR-ribotype 027 associated disease in France: preliminary report. Euro Surveill 11: E060504.1
Biller P et al. (2007) Moxifloxacin therapy as a risk factor for Clostridium difficile –associated disease during an outbreak: attempts to control a new epidemic strain. Infect Control Hosp Epidemiol 28: 198–201
Coignard B et al. (2006) Emergence of Clostridium difficile toxinotype III, PCR-ribotype 027-associated disease, France, 2006. Euro Surveill 11: E060914.1
Kazakova SV et al. (2006) A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med 166: 2518–2524
Rodriguez-Palacios A et al. (2006) Clostridium difficile PCR ribotypes in calves, Canada. Emerg Infect Dis 12: 1730–1736
Drudy D et al. (2007) Emergence and control of fluoroquinolone-resistant, toxin A-negative, toxin B-positive Clostridium difficile. Infect Control Hosp Epidemiol 28: 932–940
McFarland LV et al. (2007) Fluoroquinolone use and risk factors for Clostridium difficile disease within a Veterans Administration Health Care System. Clin Infect Dis 45: 1141–1151
Clabots CR et al. (1992) Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis 166: 561–567
Shim JK et al. (1998) Primary symptomless colonization by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet 351: 633–636
Terhes G et al. (2004) Community-acquired Clostridium difficile diarrhea caused by binary toxin, toxin A, and toxin B gene-positive isolates in Hungary. J Clin Microbiol 42: 4316–4318
Beaugerie L et al. (2003) Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment Pharmacol Ther 17: 905–912
Centers for Disease Control (2005) Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. MMWR Morb Mortal Wkly Rep 54: 1201–1205
Dial S et al. (2006) Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ 175: 745–748
Lefebvre SL et al. (2006) Prevalence of zoonotic agents in dogs visiting hospitalized people in Ontario: implications for infection control. J Hosp Infect 62: 458–466
Dial S et al. (2005) Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294: 2989–2995
Kyne L et al. (2001) Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhea. Lancet 357: 189–193
McFarland LV et al. (2000) Pediatric Clostridium difficile: a phantom menace or clinical reality. J Pediatr Gastroenterol Nutr 31: 220–231
Morinville V and McDonald J (2005) Clostridium difficile-associated diarrhea in 200 Canadian children. Can J Gastroenterol 19: 497–501
Gogate A et al. (2005) Diagnostic role of stool culture & toxin detection in antibiotic associated diarrhoea due to Clostridium difficile in children. Indian J Med Res 122: 518–524
Gaynes R et al. (2004) Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin Infect Dis 38: 640–645
Pepin J et al. (2005) Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 41: 1254–1260
Johnson S et al. (1990) Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med 88: 137–140
Gerding et al. (1995) Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol 16: 459–477
Boone N et al. (1998) Evaluation of an interdisciplinary re-isolation policy for patients with previous Clostridium difficile diarrhea. Amer J Infect. Control 26: 584–587
McMullen KM et al. (2007) Use of hypochlorite solution to decrease rates of Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol 28: 205–207
Sehulster L and Chinn RY (2003) Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 52: 1–42
Jernigan JA et al. (1998) A randomized crossover study of disposable thermometers for prevention of Clostridium difficile and other nosocomial infections. Infect Control Hosp Epidemiol 19: 494–499
Davey P et al. (2005) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Systemetic Reviews 2005, Issue 4. Art. No.: CD003543. doi:10.1002/14651858.CD003543.pub2
Dellit TH et al.; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44: 159–177
Valiquette L et al. (2007) Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease cause by the hypervirulent NAP1/027 Strain. Clin Infect Dis 45 (Suppl 2): S112–S121
Sunenshine RH and McDonald LC (2006) Clostridium difficile-associated disease: new challenges from an established pathogen. Cleveland Clinic J Med 73: 187–197
Apisarnthanarak A et al. (2004) Effectiveness of environmental and infection control programs to reduce transmission of Clostridium difficile. Clin Infect Dis 39: 601–602
Owens RC Jr and Rice L (2006) Hospital-based strategies for combating resistance. Clin Infect Dis 42 (Suppl 4): S173–S181
Zar FA et al. (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45: 302–307
McFarland LV et al. (2002) Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 97: 1769–1775
Teasley DG et al. (1983) Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhoea and colitis. Lancet 2: 1043–1046
Wenisch C et al. (1996) Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 22: 813–818
Pepin J et al. (2005) Increasing risk of relapse after treatment of Clostridium difficile colitis in Québec, Canada. Clin Infect Dis 40: 1591–1597
Musher D et al. (2005) Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 40: 1586–1590
Lagrotteria D et al. (2006) Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile-associated diarrhea. Clin Infect Dis 43: 547–552
Pelaez T et al. (2002) Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother 46: 647–1650
Louie TJ et al. (2006) Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile associated diarrhea. Clin Infect Dis 43: 411–420
Elmer GW et al. (2007) AAD and Clostridium difficile disease. In. The Power of Probiotics: Improving Your Health with Beneficial Microbes, 71–93 (Eds Elmer GE, McFarland LV, McFarland MJ) Binghamton, NY: Haworth Press
Surawicz CM et al. (2000) The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 31: 1012–1017
McFarland LV et al. (2006) Meta-analysis of probiotics for prevention of antibiotic associated diarrhea and treatment of Clostridium difficile disease. Am J Gastroenterol 101: 812–822
Mulligan ME et al. (1993) Electated levels of serum immunoglobulins in asymptomatic carriers of Clostridium difficile. Clin Infect Dis 16 (Suppl 4): S239–S244
Acknowledgements
The views expressed in this article are those of the author and do not represent the views of the Department of Veterans Affairs.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author is a speaker for Klaire Laboratory, a consultant and speaker for Laboratories Biocodex, and has received grant/research support from Massachusetts Biologic Laboratories.
Supplementary information
Supplementary Information
“Routes Amid The Chaos” Medical Abstract Series #184 by Lynne V McFarland, PhD (DOC 278 kb)
Rights and permissions
About this article
Cite this article
McFarland, L. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Rev Gastroenterol Hepatol 5, 40–48 (2008). https://doi.org/10.1038/ncpgasthep1029
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep1029
This article is cited by
-
Bifidobacterium lactis CCT 7858 Improves Gastrointestinal Symptoms by Antibiotics Treatment: a Double-Blind, Randomized, Placebo-Controlled Trial
Probiotics and Antimicrobial Proteins (2023)
-
Modeling Clostridium difficile in a hospital setting: control and admissions of colonized and symptomatic patients
Theoretical Biology and Medical Modelling (2019)
-
Use of antibiotic and prevalence of antibiotic-associated diarrhoea in-patients with spinal cord injuries: a UK national spinal injury centre experience
Spinal Cord (2017)
-
Optimal control of vaccination rate in an epidemiological model of Clostridium difficile transmission
Journal of Mathematical Biology (2017)
-
Antimicrobial Stewardship and Environmental Decontamination for the Control of Clostridium difficile Transmission in Healthcare Settings
Bulletin of Mathematical Biology (2017)