Abstract
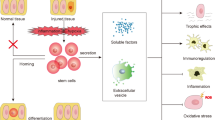
The capacity of the kidney to regenerate functional tubules following episodes of acute injury is an important determinant of patient morbidity and mortality in the hospital setting. After severe injury or repeated episodes of injury, kidney recovery can be significantly impaired or even fail completely. Although significant advances have been made in the clinical management of such cases, there is no specific therapy that can improve the rate or effectiveness of the repair process. Recent studies have indicated that adult stem cells, either in the kidney itself or derived from the bone marrow, could participate in this repair process and might therefore be utilized clinically to treat acute renal failure. This review will focus on our current understanding of these stem cells, the controversies surrounding their in vivo capacity to repopulate the renal tubule, and further investigations that will be required before stem cell therapy can be considered for use in the clinical setting.
Key Points
-
A population of tubule progenitor cells may persist in the renal interstitium of adults
-
Stem cells from the bone marrow mobilize to the kidney after injury
-
Mobilizing or infusing bone-marrow stem cells (BMSCs) can have a protectiveeffect in animal models of acute renal failure
-
It is not clear whether BMSCs differentiate to form tubule cells, or fuse with existing tubule elements
-
The low frequency of BMSC differentiation and fusion make it unlikely that these processes account for functional recovery of injured tubules
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bonventre JV (2003) Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14: S55–S61
Schena FP (1998) Role of growth factors in acute renal failure. Kidney Int 66 (Suppl): S11–S15
Vainio S and Muller U (1997) Inductive tissue interactions, cell signaling, and the control of kidney organogenesis. Cell 90: 975–978
Salice CJ et al. (2001) New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med 51: 56–59
Elger M et al. (2003) Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol 14: 1506–1518
Maeshima A et al. (2003) Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14: 3138–3146
Oliver JA et al. (2004) The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804
Cotsarelis G et al. (1989) Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57: 201–209
Goodell MA et al. (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806
Zhou S et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7: 1028–1034
Asakura A et al. (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159: 123–134
Hishikawa K et al. (2005) Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol 169: 921–928
Uchida N et al. (2000) Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 97: 14720–14725
Peichev M et al. (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952–958
Corbeil D et al. (2000) The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem 275: 5512–5520
Bussolati B et al. (2005) Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166: 545–555
Short B et al. (2003) Mesenchymal stem cells. Arch Med Res 34: 565–571
Challen GA et al. (2004) Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol 15: 2344–2357
Bensidhoum M et al. (2004) Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood 103: 3313–3319
Javazon EH et al. (2004) Mesenchymal stem cells: paradoxes of passaging. Exp Hematol 32: 414–425
Gronthos S et al. (2003) Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116: 1827–1835
Krause DS et al. (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105: 369–377
Lagasse E et al. (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6: 1229–1234
Ianus A et al. (2003) In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 111: 843–850
Reyes M et al. (2002) Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 109: 337–346
Herzog EL et al. (2003) Plasticity of marrow-derived stem cells. Blood 102: 3483–3493
Wagers AJ et al. (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297: 2256–2259
Wagers AJ and Weissman IL (2004) Plasticity of adult stem cells. Cell 116: 639–648
Kale S et al. (2003) Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49
Togel F et al. (2004) Hematopoietic stem cell mobilization-associated granulocytosis severely worsens acute renal failure. J Am Soc Nephrol 15: 1261–1267
Togel F et al. (2005) Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784
Duffield JS et al. (2005) Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755
Zhang Y et al. (2004) Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro. Am J Physiol Renal Physiol 286: F1193–F1201
Safirstein R et al. (1991) Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol 261: F1095–F1101
Guidot DM et al. (1994) Interleukin-1 treatment increases neutrophils but not antioxidant enzyme activity or resistance to ischemia-reperfusion injury in rat kidneys. Inflammation 18: 537–545
Haq M et al. (1998) Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol 9: 614–619
Patel NS et al. (2005) Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther 312: 1170–1178
Ysebaert DK et al. (2004) T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 66: 491–496
Bonventre JV and Zuk A (2004) Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485
Poulsom R et al. (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235
Gupta S et al. (2002) A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int 62: 1285–1290
Fang TC et al. (2005) Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. J Am Soc Nephrol 16: 1723–1732
Lin F et al. (2005) Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764
Anjos-Afonso F et al. (2004) In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci 117: 5655–5664
Yokoo T et al. (2005) Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci USA 102: 3296–3300
Lin F et al. (2003) Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 14: 1188–1199
Truong LD et al. (1992) Experimental chronic renal ischemia: morphologic and immunologic studies. Kidney Int 41: 1676–1689
Krause D and Cantley LG (2005) Bone marrow plasticity revisited: protection or differentiation in the kidney tubule? J Clin Invest 115: 1705–1708
Szczypka MS et al. (2005) Rare incorporation of bone marrow-derived cells into kidney after folic acid-induced injury. Stem Cells 23: 44–54
Morigi M et al. (2004) Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15: 1794–1804
Herrera MB et al. (2004) Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14: 1035–1041
Togel F et al. (2005) Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–42
Stokman G et al. (2005) Hematopoietic stem cell mobilization therapy accelerates recovery of renal function independent of stem cell contribution. J Am Soc Nephrol 16: 1684–1692
Iwasaki M et al. (2005) Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. J Am Soc Nephrol 16: 658–666
Harada M et al. (2005) G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11: 305–311
Aggarwal S and Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822
Sharples EJ et al. (2004) Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol 15: 2115–2124
Patel NS et al. (2004) Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int 66: 983–989
Acknowledgements
The author would like to thank M Egalka for the images in Figure 3, and D Krause for her helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Cantley, L. Adult stem cells in the repair of the injured renal tubule. Nat Rev Nephrol 1, 22–32 (2005). https://doi.org/10.1038/ncpneph0021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0021
This article is cited by
-
Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD
Nature Reviews Nephrology (2015)
-
The Role of Hypoxia and Cancer Stem Cells in Renal Cell Carcinoma Pathogenesis
Stem Cell Reviews and Reports (2015)
-
Extracellular vesicles derived from mesenchymal stem cells induce features of diabetic retinopathy in vitro
Acta Diabetologica (2014)
-
Pathophysiology of ischemic acute kidney injury
Nature Reviews Nephrology (2011)
-
Kidney preservation by bone marrow cell transplantation in hereditary nephropathy
Kidney International (2011)