Abstract
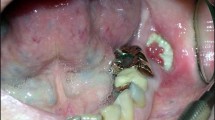
The maxillary and mandibular bones undergo high-turnover remodeling to maintain mechanical competence. Common dental or periodontal diseases can increase local bone turnover. Bisphosphonates (BPs) accumulate almost exclusively in skeletal sites that have active bone remodeling. The maxillary and mandibular bones are preferential sites for accumulation of BPs, which become buried under new layers of bone and remain biologically inactive for a long time. Surgical odontostomatological procedures create open bony wounds that heal quickly and without infection, as a result of activation of osteoclasts and subsequently osteoblasts. Once BPs are removed from the bone via activation of osteoclasts after a tooth extraction or a periodontal procedure, they induce osteoclast apoptosis. This inhibition of osteoclast bone resorption impairs bone wound healing because of decreased production of cytokines derived from the bone matrix, and the bone is exposed to the risk of osteomyelitis and necrosis. The pathogenic relationship between BPs and osteonecrosis of the jaw is unclear, but there is evidence to indicate an association between high-dose BP treatment and exposure to dental infections or oral surgical procedures. A better knowledge of the interactions between BPs and jaw and maxillary bone biology will improve clinical and therapeutic approaches.
Key Points
-
Osteonecrosis of the jaw or maxillary bone associated with bisphosphonate (BP) treatment is a relatively rare but severe clinical condition; however, a clear pathogenic relationship between osteonecrosis of the jaw and BPs has not been established
-
The jaw and the maxillary bone are characterized by high bone turnover that is constantly stimulated by mechanical stress, tooth movements or loss, periodontitis, and odontostomatological procedures, which are particularly frequent in cancer patients
-
Treatment with an amino-BP over long time periods causes the drug to accumulate in an inactive state within the alveolar bone
-
Physical trauma or infection activates local bone remodeling, releasing pharmacological doses of BPs that inhibit the osteoclast-driven bone healing
-
The nonhealing wound exposes the alveolar bone to the risk of infection by commensal flora and the wound can progress to necrotic osteomyelitis; there is no convincing evidence that a direct necrotic effect is mediated through inhibition of angiogenesis
-
The possibility of accumulation of substantial doses of BPs at skeletal sites other than those affected by bone metastases could have implications for the optimization of BP schedules to improve response in patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Santini D et al. (2003) The antineoplastic role of bisphosphonates: from basic research to clinical evidence. Ann Oncol 14: 1468–1476
Clezardin P et al. (2005) Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res 65: 4971–4974
Ross JR et al. (2003) Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 327: 469–472
Woo SB et al. (2006) Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 144: 753–761
Bamias A et al. (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23: 8580–8587
Migliorati CA et al. (2005) Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer 104: 83–93
Badros A et al. (2006) Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 24: 945–952
Mavrokokki T et al. (2007) Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 65: 415–423
Hoff A et al. (2006) Osteonecrosis of the jaw in patients receiving intravenous bisphosphonates therapy [abstract #8528]. J Clin Oncol (Suppl 24): S475
Bonafe-Oliveira L et al. (2003) Ultrastructural and histochemical examination of alveolar bone at the pressure areas of rat molars submitted to continuous orthodontic force. Eur J Oral Sci 111: 410–416
Rody WJ Jr et al. (2001) Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Dentofascial Orthop 120: 477–489
Shimizu M et al. (1998) Bone wound healing after maxillary molar extraction in ovariectomized aged rats. J Electron Microsc (Tokyo) 47: 517–526
Pitaru S et al. (1994) Cellular origins and differentiation control mechanisms during periodontal development and wound healing. J Periodontal Res 29: 81–94
Kobayashi Y et al. (2000) Forced-induced osteoclast apoptosis in vivo is accompanied by elevation in transforming growth factor β and osteoprotegerin expression. J Bone Min Res 15: 1924–1934
Terai K et al. (1999) Role of osteopontin in bone remodeling caused by mechanical stress. J Bone Miner Res 14: 839–849
Kanzaki H et al. (2002) Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor κB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 17: 210–220
Fukushima H et al. (2005) Parathyroid-hormone-related protein induces expression of receptor activator of NF-κB ligand in human periodontal ligament cells via a cAMP/protein kinase A-independent pathway. J Dent Res 84: 329–334
Tezal M et al. (2005) Periodontal disease and the incidence of tooth loss in postmenopausal women. J Periodontol 76: 1123–1128
[No authors listed] (1996) Position paper: Epidemiology of periodontal diseases. American Academy of Periodontology. J Periodontol 67: 935–945
Crotti T et al. (2003) Receptor activator NF kappa B ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res 38: 380–387
Vernal R et al. (2004) Levels of cytokine receptor activator of nuclear factor kB ligand in gingival crevicular fluid in untreated chronic periodontitis patients. J Periodontol 75: 1586–1591
Müller HP and Ulbrich M (2005) Alveolar bone levels in adults as assessed on panoramic radiographs. (I) Prevalence, extent, and severity of even and angular bone loss. Clin Oral Investig 9: 98–104
Jahangari L et al. (1998) Current perspectives in residual ridge remodeling and its clinical implication: a review. J Prosthet Dent 80: 224–237
Coxon FP et al. (2006) Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol 6: 307–312
Papapoulos SE (2006) Bisphosphonate actions: physical chemistry revisited. Bone 38: 613–616
Rogers MJ (2003) New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 9: 2643–2658
Fleisch H (1998) Bisphosphonates: mechanisms of action. Endocr Rev 19: 80–100
Roelofs AJ et al. (2006) Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res 12 (Suppl 20): S6222–S6230
Coxon FP et al. (2005) Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone 37: 349–358
Reid IR et al. (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346: 653–661
Reid IR et al. (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget's disease. N Engl J Med 353: 898–908
Black D et al. (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-Term EXtension (FLEX): a randomized trial. J Am Med Ass 296: 2927–2938
Watts NB et al. (2007) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int [doi:10.1007/s00198-007-0460-7]
Landman JO et al. (1995) Skeletal metabolism in patients with osteoporosis after discontinuation of long-term treatment with oral pamidronate. J Clin Endocrinol Metab 80: 3465–3468
Masarachia P et al. (1996) Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone 19: 281–290
Cremers S et al. (2005) Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 44: 551–570
Berenson JR et al. (1997) Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 37: 285–290
Cremers SC et al. (2003) Relationships between pharmacokinetics and rate of bone turnover after intravenous bisphosphonate (olpadronate) in patients with Paget's disease of bone. J Bone Miner Res 18: 868–875
Cremers SC et al. (2005) Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res 20: 1543–1547
Altundal H and Guvener O (2004) The effect of alendronate on resorption of the alveolar bone following tooth extraction. Int J Oral Maxillofac Surg 33: 286–293
Weireb M et al. (1994) Istomorphometrical analysis of the effects of the bisphosphonates alendronate on bone loss caused by experimental periodontitis in monkeys. J Periodontal Res 29: 35–40
Rody WJ et al. (2001) Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Denofacial Orthop 120: 477–489
Shell H et al. (2006) Osteoclast activity begins early and increase over the course of bone healing. Bone 38: 547–554
Linkhart TA et al. (1996) Growth factors for bone growth and repair: IGF, TGF-beta, and BMP. Bone 19 (Suppl 1): S1–S12
Cho TJ et al. (2002) Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res 17: 513–520
Lalani Z et al. (2003) Spatial and temporal localization of transforming growth factor-beta1, bone morphogenetic protein–2, and platelet-derived growth factor-A in healing tooth extraction sockets in a rabbit model. J Oral Maxillofac Surg 61: 1061–1072
Uematsu S et al. (1997) Increase of transforming growth factor-beta 1 in gingival crevicular fluid during human orthodontic tooth movement. Arch Oral Biol 41: 1091–1095
Götz W et al. (2006) Insulin-like growth factor system components in the periodontium during tooth root resorption and early repair process in the rat. Eur J Oral Sci 114: 318–327
Van den Wyngaert T et al. (2006) Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol 17: 1197–1204
Ruggiero S et al. (2006) Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract 2: 7–14
Bilston LE et al. (2002) Zoledronic acid improves the mechanical properties of normal and healing bone. Clin Biomech (Bristol, Avon) 17: 716–718
Mashiba T et al. (2001) Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone 28: 524–531
Cao Y et al. (2002) Raloxifene, estrogen and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17: 2237–2246
Goodship AE et al. (1994) Use of a bisphosphonate (pamidronate) to modulate fracture repair in ovine bone. Ann Oncol 5 (Suppl 7): S53–S55
Hansen T et al. (2006) Osteonecrosis of the jaws in patients treated with bisphosphonates—histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med 35: 155–160
Sung EC et al. (2002) Osteonecrosis of the maxilla as a complication to chemotherapy: a case report. Spec Care Dentist 22: 142–146
Hino S et al. (2005) Response of diffuse sclerosing osteomyelitis of the mandible to alendronate: follow-up study by 99mTc scintigraphy. Int J Oral Maxillofac Surg 34: 576–578
Ruggiero SL et al. (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62: 527–534
Hellstein JW and Marek CL (2005) Bisphosphonate osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg 63: 682–689
Assael LA (2004) New foundations in understanding osteonecrosis of the jaws. J Oral Maxillofac Surg 62: 125–126
Robinson JN et al. (2004) Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med 5: 276–280
Wood J et al. (2002) Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 302: 1055–1061
Vincenzi B et al. (2005) Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res 25: 144–151
Fournier P et al. (2002) Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 62: 6538–6544
Santini D et al. (2002) Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res 8: 1080–1084
Deckers MM et al. (2002) Dissociation of angiogenesis and osteoclastogenesis during endochondral bone formation in neonatal mice. J Bone Miner Res 17: 998–1007
Heimdahl A (1999) Prevention and management of oral infections in cancer patient. Support Care Cancer 7: 224–228
Sonis ST (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34: 39–43
Raber-Durlacher JE et al. (2002) Periodontal infection in cancer patients treated with high-dose chemotherapy. Support Care Cancer 10: 466–473
Tiranathanagul S et al. (2004) Actinobacillus actinomycetemcomitans lipopolysaccharide activates matrix metalloproteinase-2 and increases receptor activator of nuclear factor-κB ligand expression in human periodontal ligament cells. J Periodontol 75: 1647–1654
Narani N and Epstein JB (2001) Classifications of oral lesions in HIV infection. J Clin Periodontol 28: 137–145
Dunford JE et al. (2001) Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296: 235–242
Green JR et al. (1994) Preclinical pharmacology of CGP 42'446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res 9: 745–751
Rosen CJ et al. (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double blind study. J Bone Miner Res 20: 141–151
Barret J et al. (2004) Ibandronate: a clinical pharmacological and pharmacokinetic update. J Clin Pharmacol 44: 951–965
Gertz BJ et al. (1995) Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther 58: 288–298
Coleman RE (2005) Bisphosphonates in breast cancer. Ann Oncol 16: 687–695
Major PP and Cook R (2002) Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol (Suppl 1): S10–S18
Body JJ et al. (2004) Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: result from two randomised, placebo-controlled phase III studies. Br J Cancer 90: 1133–1137
Dimopoulos MA et al. (2006) Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 9: 968–971
Van den Wyngaer T et al. (2006) Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol 17: 1197–1204
Bilezikian JP (2006) Osteonecrosis of the jaw: do bisphosphonates pose a risk? N Engl J Med 355: 2278–2281
Christiansen C et al. (2003) Comparison of risedronate and alendronate pharmacokinetics at clinical doses [abstract #P163]. Osteoporosis Int 14 (Suppl 7): S38
Black DM et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Eng J Med 356: 1809–1822
Brown JE et al. (2003) Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 89: 2031–2037
Coleman RE et al. (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23: 4925–4935
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bertoldo, F., Santini, D. & Lo Cascio, V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Rev Clin Oncol 4, 711–721 (2007). https://doi.org/10.1038/ncponc1000
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1000
This article is cited by
-
Inhibition of osteoclastogenesis after bisphosphonate therapy discontinuation: an in vitro approach
Journal of Molecular Histology (2022)
-
Effect of bisphosphonate treatment on the jawbone: an exploratory study using periapical and panoramic radiographic evaluation
Oral Radiology (2019)
-
Bisphosphonate Related Osteonecrosis of the Jaw: An Update
Journal of Maxillofacial and Oral Surgery (2014)
-
The phosphorous necrosis of the jaws and what can we learn from the past: a comparison of “phossy” and “bisphossy” jaw
Oral and Maxillofacial Surgery (2014)
-
A brief review: characteristics of bisphosphonate-related osteonecrosis of the jaw (BRONJ) from the viewpoint of pathology
Oral Radiology (2013)