Abstract
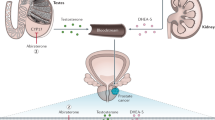
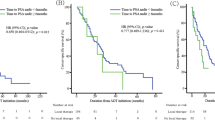
An estimated 20–40% of men experience a biochemical recurrence within 10 years of definitive prostate cancer treatment. No single prostate-specific antigen (PSA) value is invariably associated with clinical metastasis or cancer-specific survival; PSA kinetics might prove to be a more important predictor of eventual progression-free survival and cancer-specific survival than absolute PSA level alone. With only one-third of patients progressing from biochemical recurrence to clinical disease, therapeutic morbidity should not outpace risk of disease progression. Salvage radiation therapy following radical prostatectomy has widely variable long-term biochemical control rates (from 18 to 64% depending on the follow-up period). Early hormonal therapy delivered as castration or complete androgen blockade might delay clinical metastasis in patients with high-risk pathologic disease; however, the adverse effects and morbidity of long-term therapy must not be underestimated. Non-steroidal antiandrogens as monotherapy for early biochemical recurrence, particularly for younger men who wish to preserve their libido and sexual potency, have received considerable attention, but there are conflicting data on long-term outcomes. Because of their favorable adverse-effect profiles, non-traditional therapies that exert localized hormonal or cellular effects are receiving considerable attention for treatment of early, PSA-only recurrence. Data from animal models provide a rationale for the use of these therapies, but there is a lack of evidence to support prolongation of progression-free survival or cancer-specific survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Khan MA et al. (2003) Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology 62: 86–91; discussion 91–92
Djavan B et al. (2003) PSA progression following radical prostatectomy and radiation therapy: new standards in the new Millennium. Eur Urol 43: 12–27
Polascik TJ et al. (1999) Prostate specific antigen: a decade of discovery—what we have learned and where we are going. J Urol 162: 293–306
Ward JF et al. (2003) The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 170: 1872–1876
Pound CR et al. (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281: 1591–1597
Amling CL et al. (2001) Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol 165: 1146–1151
Freedland SJ et al. (2003) Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology 61: 365–369
American Society for Therapeutic Radiology and Oncology Consensus Panel (1997) Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys 37: 1035–1041
Taylor JM et al. (2001) Definitions of biochemical failure in prostate cancer following radiation therapy. Int J Radiat Oncol Biol Phys 50: 1212–1219
Thames H et al. (2003) Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys 57: 929–943
Pruthi RS et al. (1997) Prostate-specific antigen doubling times in patients who have failed radical prostatectomy: correlation with histologic characteristics of the primary cancer. Urology 49: 737–742
D'Amico AV et al. (2003) Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 95: 1376–1383
Albertsen PC et al. (2004) Validation of increasing prostate specific antigen as a predictor of prostate cancer death after treatment of localized prostate cancer with surgery or radiation. J Urol 171: 2221–2225
D'Amico AV et al. (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280: 969–974
[no author listed] (online 1 July 2004) Prostate Cancer End Points Workshop Presentations (accessed 25 February 2005)
Leventis AK et al. (2001) Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol 19: 1030–1039
Katz MS et al. (2003) Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol 21: 483–489
Song DY et al. (2002) Salvage radiotherapy for rising or persistent PSA after radical prostatectomy. Urology 60: 281–287
Anscher MS et al. (2000) Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys 48: 369–375
Pisansky TM et al. (2000) Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol 163: 845–850
Chawla AK et al. (2002) Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: analysis of efficacy and prognostic factors. Urology 59: 726–731
Cadeddu JA et al. (1998) Long-term results of radiation therapy for prostate cancer recurrence following radical prostatectomy. J Urol 159: 173–177; discussion 177–178
Cox JD et al. (1999) Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol 17: 1155
Schild SE et al. (1996) The use of radiotherapy for patients with isolated elevation of serum prostate specific antigen following radical prostatectomy. J Urol 156: 1725–1729
Ward JF et al. (2004) Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol 172: 2244–2248
Stephenson AJ et al. (2004) Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 291: 1325–1332
Kaplan ID et al. (1992) The importance of local control in the treatment of prostatic cancer. J Urol 147: 917–921
Crook JM et al. (1995) Routine prostate biopsies following radiotherapy for prostate cancer: results for 226 patients. Urology 45: 624–631; discussion 631–632
Ward JF et al. (2005) Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol 173: 1156–1160
Gheiler EL (1998) Predictors for maximal outcome in patients undergoing salvage surgery for radio-recurrent prostate cancer. Urology 51: 789–795
Garzotto M and Wajsman Z (1998) Androgen deprivation with salvage surgery for radiorecurrent prostate cancer: results at 5-year followup. J Urol 159: 950–954; discussion 954–955
Rogers E et al. (1995) Salvage radical prostatectomy: outcome measured by serum prostate specific antigen levels. J Urol 153: 104–110
Stephenson AJ et al. (2004) Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol 172: 2239–2243
Katz AE and Rukstalis DB (2002) Introduction. Recent scientific and technological advances have challenged the traditional treatment options for patients with localized prostate cancer. Urology 60: 1–2
Benoit RM et al. (2000) Cryosurgery for prostate cancer: new technology and indications. Curr Urol Rep 1: 41–47
Han KR et al. (2003) Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multicenter experience. J Urol 170: 1126–1130
Wei JT et al. (2002) Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 20: 557–566
The Medical Research Council Prostate Cancer Working Party Investigators Group (1997) Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol 79: 235–246
Crawford ED et al. (1989) A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321: 419–424
Denis LJ et al. (1998) Maximal androgen blockade: final analysis of EORTC phase III trial 30853. EORTC Genito-Urinary Tract Cancer Cooperative Group and the EORTC Data Center. Eur Urol 33: 144–151
Eisenberger MA et al. (1998) Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339: 1036–1042
Schellhammer PF et al. (1997) Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group. Urology 50: 330–336
Moul JW et al. (2004) Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol 171: 1141–1147
Kirk D (2000) Immediate vs. delayed hormone treatment for prostate cancer: how safe is androgen deprivation? BJU Int 86 (Suppl 3): 220
Verhelst J et al. (1994) Endocrine profiles during administration of the new non-steroidal anti-androgen Casodex in prostate cancer. Clin Endocrinol (Oxf) 41: 525–530
Brufsky A et al. (1997) Finasteride and flutamide as potency-sparing androgen-ablative therapy for advanced adenocarcinoma of the prostate. Urology 49: 913–920
Tyrrell CJ et al. (2003) The non-steroidal antiandrogen, bicalutamide ('Casodex'), may preserve bone mineral density as compared with castration: results of a preliminary study. World J Urol 21: 37–42
Reese DM (2000) Choice of hormonal therapy for prostate cancer. Lancet 355: 1474–1475
Kaisary AV et al. (2001) Is there a role for antiandrogen monotherapy in patients with metastatic prostate cancer? Prostate Cancer Prostatic Dis 4: 196–203
Bales GT and Chodak GW (1996) A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 47: 38–43; discussion 48–53
Tyrrell CJ et al. (1998) A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol 33: 447–456
Iversen P et al. (2000) Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol 164: 1579–1582
Boccardo F et al. (2002) Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer: updated results of a multicentric trial. Eur Urol 42: 481–490
See WA et al. (2001) The bicalutamide Early Prostate Cancer Program. Demography. Urol Oncol 6: 43–47
See WA et al. (2002) Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the early prostate cancer program. J Urol 168: 429–435
Wirth MP et al. (2004) Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. J Urol 172: 1865–1870
Iversen P et al. (2004) Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6. J Urol 172: 1871–1876
Andriole G et al. (1995) Treatment with finasteride following radical prostatectomy for prostate cancer. Urology 45: 491–497
Fleshner NE and Trachtenberg J (1995) Combination finasteride and flutamide in advanced carcinoma of the prostate: effective therapy with minimal side effects. J Urol 154: 1642–1645; discussion 1645–1646
Ornstein DK et al. (1996) Combined finasteride and flutamide therapy in men with advanced prostate cancer. Urology 48: 901–905
Oh WK et al. (2003) Finasteride and flutamide therapy in patients with advanced prostate cancer: response to subsequent castration and long-term follow-up. Urology 62: 99–104
Zaccheo T et al. (2000) Combined treatment of Dunning R3327 rat prostatic tumor with the 5alpha-reductase inhibitor PNU 157706 and the antiandrogen bicalutamide. Cancer Chemother Pharmacol 45: 31–37
Tsukamoto S et al. (1998) A five-alpha reductase inhibitor or an antiandrogen prevents the progression of microscopic prostate carcinoma to macroscopic carcinoma in rats. Cancer 82: 531–537
Pruthi RS et al. (2004) A pilot study of use of the cyclooxygenase-2 inhibitor celecoxib in recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. BJU Int 93: 275–278
Goluboff ET et al. (1999) Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology 53: 440–445
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as reflective of the views or policies of the US Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- GLEASON GRADE
-
The grade assigned to each of the two largest cancerous areas of tissue samples; grades range from 1 (least aggressive) to 5 (most aggressive)
- GLEASON SCORE
-
Sum of grades assigned to the two largest cancerous areas of tissue samples; grades range from 1 (least aggressive) to 5 (most aggressive)
Rights and permissions
About this article
Cite this article
Ward, J., Moul, J. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Rev Urol 2, 174–182 (2005). https://doi.org/10.1038/ncpuro0145
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0145
This article is cited by
-
Phase III randomised controlled trial on PSMA PET/CT guided hypofractionated salvage prostate bed radiotherapy of biochemical failure after radical prostatectomy for prostate cancer (PERYTON-trial): study protocol
BMC Cancer (2022)
-
Imaging gastrin-releasing peptide receptors (GRPRs) in prostate cancer
Clinical and Translational Imaging (2019)
-
Salvage radiotherapy for macroscopic local recurrences after radical prostatectomy
Strahlentherapie und Onkologie (2018)
-
Use of androgen deprivation and salvage radiation therapy for patients with prostate cancer and biochemical recurrence after prostatectomy
Strahlentherapie und Onkologie (2018)
-
Risk adapted dose-intensified postoperative radiation therapy in prostate cancer patients using a simultaneous integrated boost technique applied with helical Tomotherapy
Radiation Oncology (2017)